Poster Session C
Myopathic rheumatic diseases (polymyositis, dermatomyositis, inclusion body myositis)
Session: (1945–1972) Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster III
1945: Case Series of 6 Patients Exhibiting Myositis as a Rheumatologic Adverse Events Related to Cancer Immunotherapy in Two Spanish Hospitals
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- AG
Antonio Gomez-Centeno, MD
Parc Taulí Hospital Universitari
Sabadell, SpainDisclosure information not submitted.
Abstract Poster Presenter(s)
Arturo Llobell1, Inigo Gonzalez-Mazon2, carmen secada3, Adrian Martin-Gutierrez3, Almudena García-Castaño3, Enrique Gallardo4, Soledad Retamozo5, Antonio Gomez-Centeno6, Luis Fernandez-Morales4, Jordi Gratacos Masmitja7 and Ricardo Blanco8, 1Parc Tauli University Hospital, Barcelona, Spain, 2Hospital Universitario Marques de Valdecilla, Santander, Spain, 3Hospital Universitario Marqués de Valdecilla, Santander, Spain, 4Parc Taulí University Hospital, Sabadell, Spain, 5Laboratory of Autoimmune Diseases Josep Font, IDIBAPS-CELLEX, Barcelona, Spain, 6Parc Taulí Hospital Universitari, Sabadell, Spain, 7University Hospital Parc Taulí, Sabadell, Spain, 8Hospital Universitario Marqués de Valdecilla, IDIVAL, Santander, Spain
Background/Purpose: Myositis is a rare inflammatory complication associated with immune related adverse events (irAE) of immune checkpoints inhibition (ICI) immunotherapy1. It is characterized by musculoskeletal inflammation, resulting in significant muscle weakness and pain. Diagnosis is based on clinical evaluations, blood tests, and imaging studies. Management of myositis may involve the suspension or adjustment of immunotherapy, as well as the administration of anti-inflammatory or immunosuppressive medications2. Although this complication can be severe, immunotherapy remains a valuable tool in cancer treatment for these patients.
Methods: A retrospective, descriptive study of patients treated with ICIs that developed myositis as a result of said treatment, at Parc Taulí and Marqués de Valdecilla University Hospitals, between 2016 and 2022. Medical records were reviewed by a rheumatologist to identify demographic and clinic relevant data.
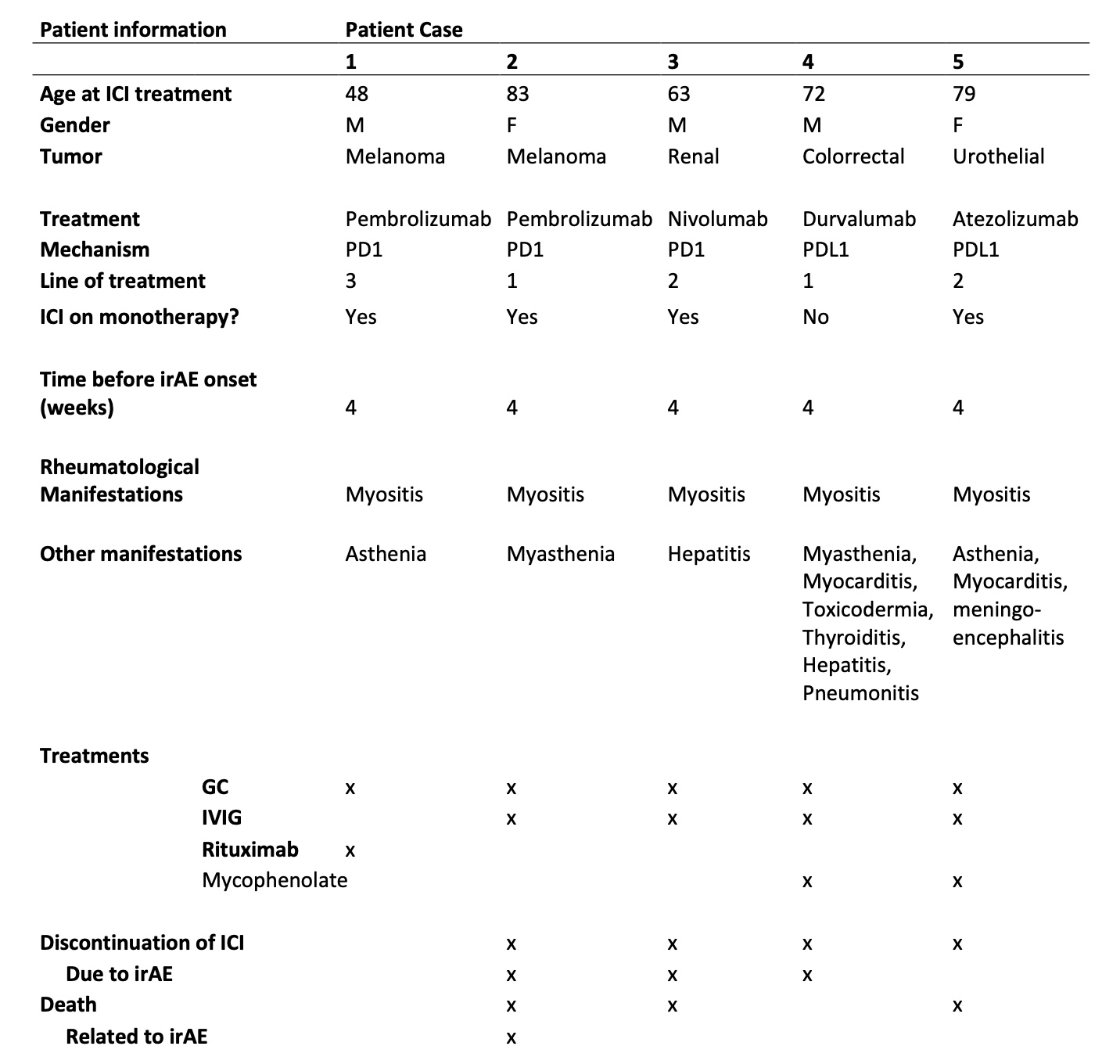
Results: From a cohort of 734 patients treated with ICIs, 54 presented rheumatologic irAEs, of which 5 developed myositis, (3 male/2 female). 3 were treated with anti-PD1, 2 with anti-PDL1. Underlying cancer types included melanoma (n=2), colorectal (n=1), renal (n=1) and urothelial (n=1). Patients presented concomitant irAEs, such as myasthenia (2), hepatitis (2), pneumonitis (1), meningoencephalitis (1) and myocarditis (1). All patients required treatment with high doses of glucocorticoids and the association of either intravenous immunoglobulins (IGIV) (n=4) and/or biologic (n=1) or synthetic (n=2) immunosuppressant (IS) drugs. In 4 out of 5 cases, this irAE was the main reason for ICI treatment discontinuation, and only 1 presented permanent response to treatment. Table 1 shows the patients' clinical characteristics, presentation, treatments and outcomes. Serologically, only 1 patient presented elevated CRP, but 4 out of 5 presented positivity for autoantibodies, which included anti-Ro52 anti-PM Scl, ANA Nucleolar pattern, anti-GAD and anti-SRP.
Conclusion: Our study highlights the significant burden of irAE myositis in patients undergoing ICI therapy. All 5 patients in our study required intensive treatment with corticosteroids, 4/5 received intravenous immunoglobulin (IVIG), and 4/5 tested positive for autoantibodies and presented other irAEs. There was only complete response to the treatment of the irAE on 1 patient, with the remaining 5 requiring maintenance treatment and experiencing relapses or little improvement. 3 patients died, at least in one of them by irAE related complications. These findings emphasize the need for increased vigilance in monitoring and early intervention to manage irAEs such as myositis effectively. Further research is needed to understand the underlying mechanisms and improve detection and treatment strategies for this complication of immunotherapy.
Bibliography:
1.- Postow MA et al. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. NEJM. 2018 Jan 11;378(2):158-168.
2.- Boutros A et al. Neuromuscular and cardiac adverse events associated with immune checkpoint inhibitors: pooled analysis of individual cases from multiple institutions and literature. ESMO Open. 2023 Feb;8(1):100791.
A. Llobell: None; I. Gonzalez-Mazon: None; c. secada: None; A. Martin-Gutierrez: None; A. García-Castaño: None; E. Gallardo: None; S. Retamozo: None; A. Gomez-Centeno: None; L. Fernandez-Morales: None; J. Gratacos Masmitja: AbbVie/Abbott, 1, 6, Amgen, 6, AstraZeneca, 6, Bristol-Myers Squibb(BMS), 6, Eli Lilly, 1, 6, Janssen, 1, 6, Novartis, 1, 6, Pfizer, 1, 6, UCB, 1, 6; R. Blanco: AbbVie, 5, 6, Amgen, 6, AstraZeneca, 2, BMS, 6, Eli Lilly, 6, Galapagos, 2, 6, Janssen, 2, 6, MSD, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche, 5, 6, Sanofi, 6.
Background/Purpose: Myositis is a rare inflammatory complication associated with immune related adverse events (irAE) of immune checkpoints inhibition (ICI) immunotherapy1. It is characterized by musculoskeletal inflammation, resulting in significant muscle weakness and pain. Diagnosis is based on clinical evaluations, blood tests, and imaging studies. Management of myositis may involve the suspension or adjustment of immunotherapy, as well as the administration of anti-inflammatory or immunosuppressive medications2. Although this complication can be severe, immunotherapy remains a valuable tool in cancer treatment for these patients.
Methods: A retrospective, descriptive study of patients treated with ICIs that developed myositis as a result of said treatment, at Parc Taulí and Marqués de Valdecilla University Hospitals, between 2016 and 2022. Medical records were reviewed by a rheumatologist to identify demographic and clinic relevant data.
Results: From a cohort of 734 patients treated with ICIs, 54 presented rheumatologic irAEs, of which 5 developed myositis, (3 male/2 female). 3 were treated with anti-PD1, 2 with anti-PDL1. Underlying cancer types included melanoma (n=2), colorectal (n=1), renal (n=1) and urothelial (n=1). Patients presented concomitant irAEs, such as myasthenia (2), hepatitis (2), pneumonitis (1), meningoencephalitis (1) and myocarditis (1). All patients required treatment with high doses of glucocorticoids and the association of either intravenous immunoglobulins (IGIV) (n=4) and/or biologic (n=1) or synthetic (n=2) immunosuppressant (IS) drugs. In 4 out of 5 cases, this irAE was the main reason for ICI treatment discontinuation, and only 1 presented permanent response to treatment. Table 1 shows the patients' clinical characteristics, presentation, treatments and outcomes. Serologically, only 1 patient presented elevated CRP, but 4 out of 5 presented positivity for autoantibodies, which included anti-Ro52 anti-PM Scl, ANA Nucleolar pattern, anti-GAD and anti-SRP.
Conclusion: Our study highlights the significant burden of irAE myositis in patients undergoing ICI therapy. All 5 patients in our study required intensive treatment with corticosteroids, 4/5 received intravenous immunoglobulin (IVIG), and 4/5 tested positive for autoantibodies and presented other irAEs. There was only complete response to the treatment of the irAE on 1 patient, with the remaining 5 requiring maintenance treatment and experiencing relapses or little improvement. 3 patients died, at least in one of them by irAE related complications. These findings emphasize the need for increased vigilance in monitoring and early intervention to manage irAEs such as myositis effectively. Further research is needed to understand the underlying mechanisms and improve detection and treatment strategies for this complication of immunotherapy.
Bibliography:
1.- Postow MA et al. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. NEJM. 2018 Jan 11;378(2):158-168.
2.- Boutros A et al. Neuromuscular and cardiac adverse events associated with immune checkpoint inhibitors: pooled analysis of individual cases from multiple institutions and literature. ESMO Open. 2023 Feb;8(1):100791.

Table 1: Patients clinical characteristics, presentation, treatments and outcomes.
A. Llobell: None; I. Gonzalez-Mazon: None; c. secada: None; A. Martin-Gutierrez: None; A. García-Castaño: None; E. Gallardo: None; S. Retamozo: None; A. Gomez-Centeno: None; L. Fernandez-Morales: None; J. Gratacos Masmitja: AbbVie/Abbott, 1, 6, Amgen, 6, AstraZeneca, 6, Bristol-Myers Squibb(BMS), 6, Eli Lilly, 1, 6, Janssen, 1, 6, Novartis, 1, 6, Pfizer, 1, 6, UCB, 1, 6; R. Blanco: AbbVie, 5, 6, Amgen, 6, AstraZeneca, 2, BMS, 6, Eli Lilly, 6, Galapagos, 2, 6, Janssen, 2, 6, MSD, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche, 5, 6, Sanofi, 6.



