Poster Session C
Rheumatoid arthritis (RA)
Session: (1734–1775) RA – Etiology and Pathogenesis Poster
1734: Serum PDGF-BB Levels Correlate with Lung Fibrosis in Mice Injected with Malondialdehyde-Acetaldehyde and/or Citrulline Modified Vimentin
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
.jpg)
Nozima Aripova, BS
University of Nebraska Medical Center
Omaha, NE, United StatesDisclosure information not submitted.
Abstract Poster Presenter(s)
Nozima Aripova1, Michael Duryee1, Carlos Hunter1, Amy Nelson1, Breanna Butler1, Jill Poole1, Bryant England1, Geoffrey Thiele1 and Ted R Mikuls2, 1University of Nebraska Medical Center, Omaha, NE, 2Division of Rheumatology and Immunology, University of Nebraska Medical Center, Omaha, NE
Background/Purpose: Pulmonary manifestations of rheumatoid arthritis (RA), such as interstitial lung disease (RA-ILD), are a major contributor to morbidity and mortality. The mechanism of pulmonary fibrosis occurring in RA-ILD is not well understood. Lung tissues from patients with RA-ILD show increased expression of vimentin (VIM) and co-localization of citrulline (CIT) and malondialdehyde-acetaldehyde (MAA) modifications. Fibrotic lung disease such as RA-ILD involves increased cellular proliferation and excessive production of extracellular matrix proteins. Such cellular responses in fibrotic lung disease are modulated by a variety of cytokines, chemokines, and growth factors. One such growth factor, platelet-derived growth factor (PDGF)-BB has been strongly associated with tissue fibrosis but has been the subject of limited studies in RA-ILD. Therefore, the current study aimed to determine whether arthritis-prone (DBA/1J) mice immunized with MAA- and/or CIT-modified VIM develop pro-fibrotic responses in the lungs and altered PDGF-BB levels in the lungs or serum.
Methods: Arthritis-prone male (DBA/1J) mice (n=5/group) were given weekly subcutaneous injections at 25μg/mL (for VIM antigens)with a) saline, b) native VIM, c) MAA-modified VIM (VIM-MAA), d) CIT-modified VIM (VIM-CIT), or e) both (VIM-MAA-CIT) for 5 weeks. At week 6, mice were euthanized, and lung tissues were stained with trichrome for collagen deposition to assess fibrotic lung responses. Additionally, serum and lung tissue homogenates were collected for quantification of PDGF-BB levels using ELISA. Group differences were compared using one-way ANOVA and Bonferroni multiple comparison test.
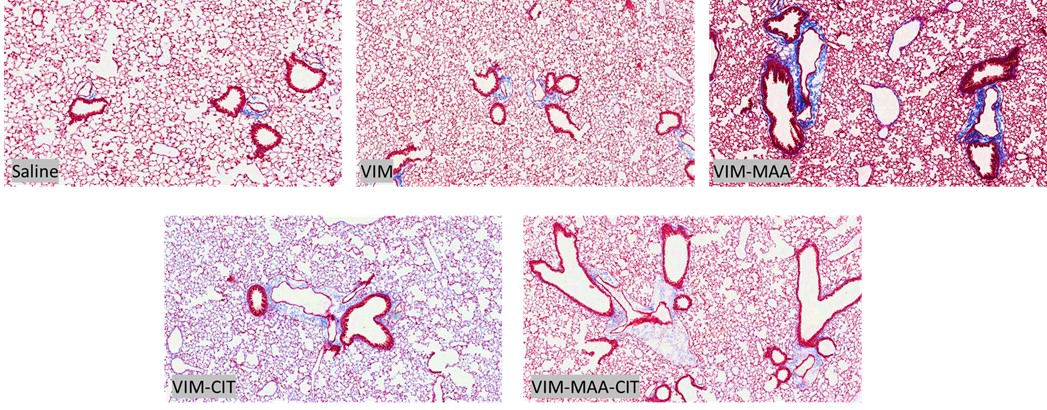
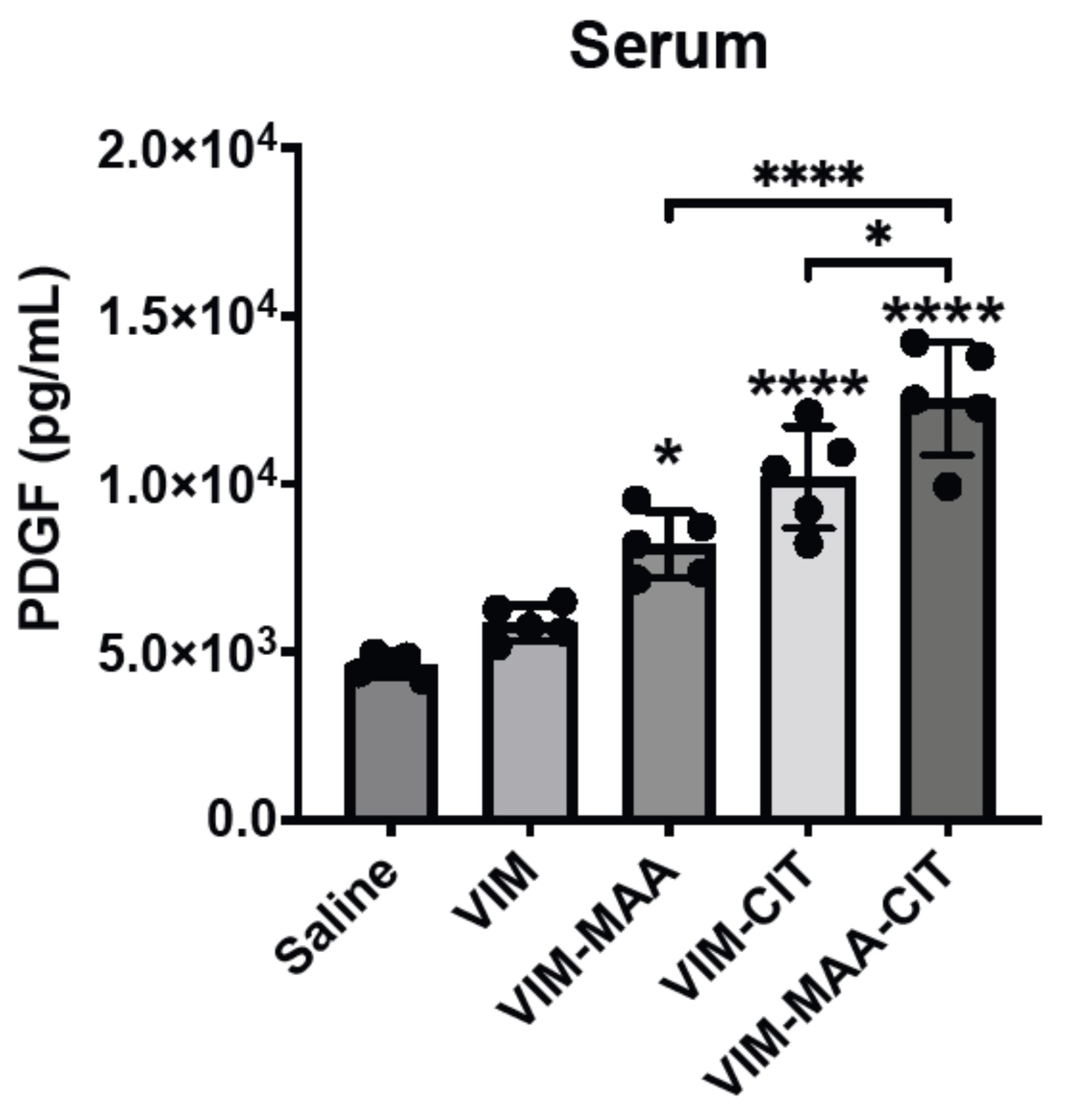
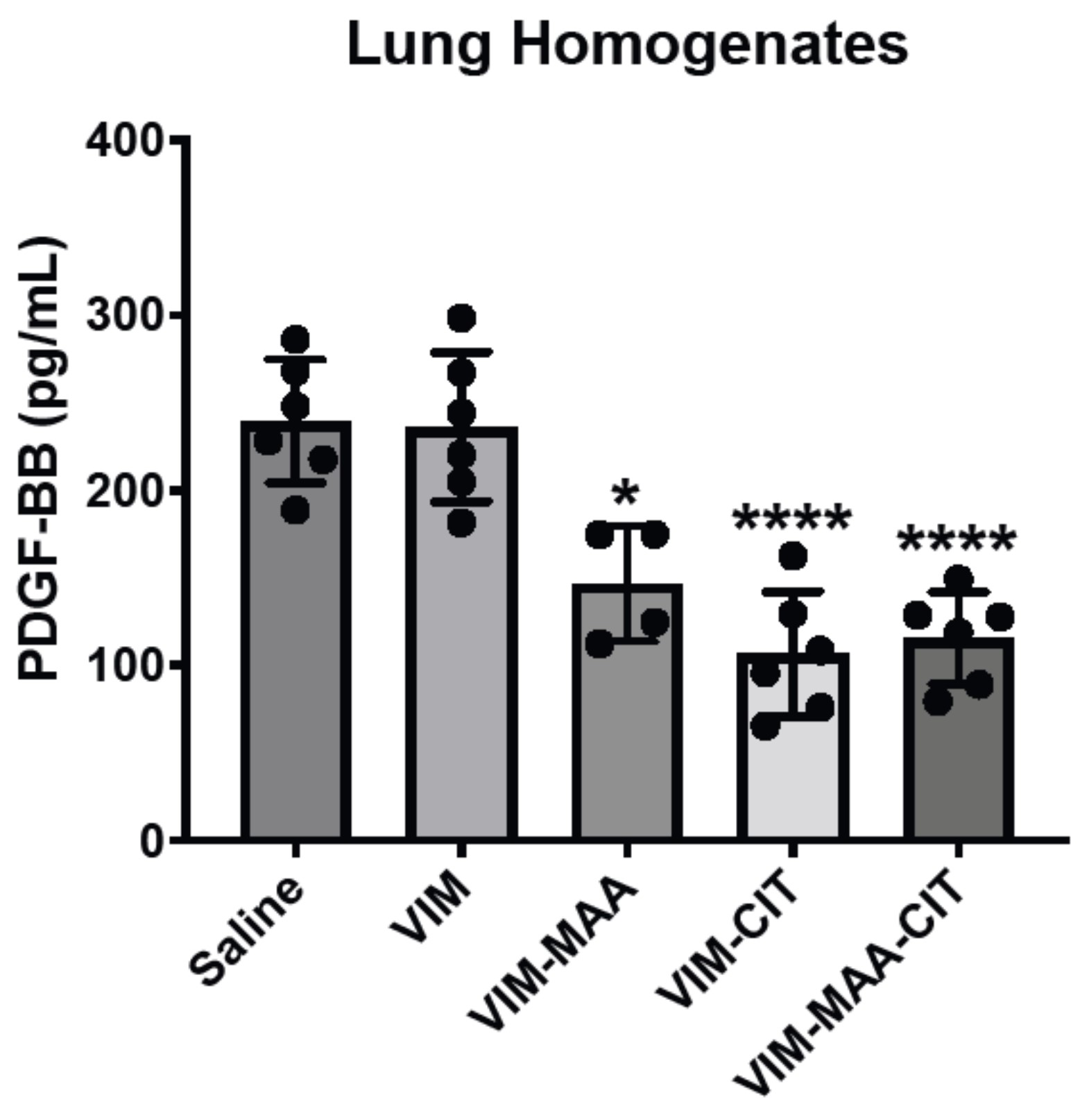
Results: Trichrome staining of lungs demonstrated increased collagen deposition in mice immunized with modified VIM vs. native VIM (Fig.1). Serum PDGF-BB levels (mean±SEM) were highest in VIM-MAA-CIT injected group (1.3x104±1.1x103; p< 0.05 vs. others), and significantly higher than in mice immunized with VIM-CIT (p< 0.0001) or VIM-MAA (p< 0.05) alone (Fig.2). In the lung homogenates, PDGF-BB levels were decreased significantly in VIM-MAA-CIT (115.3±10.8; p< 0.0001 vs. VIM), VIM-CIT (106±14.6; p< 0.0001), and VIM-MAA (146.5±16.4; p< 0.05) compared to VIM and no significant differences between treatment groups were detected (Fig.3).
Conclusion: Immunization of arthritis-prone mice with modified vimentin leads to collagen deposition in lung tissues, suggesting that systemic immune reactivity to these antigens could a play in the pathogenesis of pulmonary fibrosis. In addition to generating collagen deposition, immunization of mice with dually-modified vimentin demonstrated the highest serum PDGF-BB levels compared to either single modification. In contrast, lung homogenates of mice immunized with modified vimentin showed a reduction in PDGF-BB levels compared to native vimentin. These findings suggest that PDGF-BB is either released into the circulation resulting in increased serum levels or consumed/bound by fibroblasts in the lungs of mice immunized with modified vimentin. Thus, PDGF-BB may serve as an important mediator between post-translational modifications of vimentin and fibrotic lung disease (i.e. RA-ILD).
N. Aripova: None; M. Duryee: None; C. Hunter: None; A. Nelson: None; B. Butler: None; J. Poole: AstraZeneca, 12, I have received no monies. I received anti-IL-33 monoclonal antibody from AstraZeneca for animal research sutdies.; B. England: Boehringer-Ingelheim, 2, 5; G. Thiele: None; T. Mikuls: Elsevier, 9, Horizon Therapeutics, 2, 5, Pfizer, 2, Sanofi, 2, UCB Pharma, 2, Wolters Kluwer Health (UpToDate), 9.
Background/Purpose: Pulmonary manifestations of rheumatoid arthritis (RA), such as interstitial lung disease (RA-ILD), are a major contributor to morbidity and mortality. The mechanism of pulmonary fibrosis occurring in RA-ILD is not well understood. Lung tissues from patients with RA-ILD show increased expression of vimentin (VIM) and co-localization of citrulline (CIT) and malondialdehyde-acetaldehyde (MAA) modifications. Fibrotic lung disease such as RA-ILD involves increased cellular proliferation and excessive production of extracellular matrix proteins. Such cellular responses in fibrotic lung disease are modulated by a variety of cytokines, chemokines, and growth factors. One such growth factor, platelet-derived growth factor (PDGF)-BB has been strongly associated with tissue fibrosis but has been the subject of limited studies in RA-ILD. Therefore, the current study aimed to determine whether arthritis-prone (DBA/1J) mice immunized with MAA- and/or CIT-modified VIM develop pro-fibrotic responses in the lungs and altered PDGF-BB levels in the lungs or serum.
Methods: Arthritis-prone male (DBA/1J) mice (n=5/group) were given weekly subcutaneous injections at 25μg/mL (for VIM antigens)with a) saline, b) native VIM, c) MAA-modified VIM (VIM-MAA), d) CIT-modified VIM (VIM-CIT), or e) both (VIM-MAA-CIT) for 5 weeks. At week 6, mice were euthanized, and lung tissues were stained with trichrome for collagen deposition to assess fibrotic lung responses. Additionally, serum and lung tissue homogenates were collected for quantification of PDGF-BB levels using ELISA. Group differences were compared using one-way ANOVA and Bonferroni multiple comparison test.
Results: Trichrome staining of lungs demonstrated increased collagen deposition in mice immunized with modified VIM vs. native VIM (Fig.1). Serum PDGF-BB levels (mean±SEM) were highest in VIM-MAA-CIT injected group (1.3x104±1.1x103; p< 0.05 vs. others), and significantly higher than in mice immunized with VIM-CIT (p< 0.0001) or VIM-MAA (p< 0.05) alone (Fig.2). In the lung homogenates, PDGF-BB levels were decreased significantly in VIM-MAA-CIT (115.3±10.8; p< 0.0001 vs. VIM), VIM-CIT (106±14.6; p< 0.0001), and VIM-MAA (146.5±16.4; p< 0.05) compared to VIM and no significant differences between treatment groups were detected (Fig.3).
Conclusion: Immunization of arthritis-prone mice with modified vimentin leads to collagen deposition in lung tissues, suggesting that systemic immune reactivity to these antigens could a play in the pathogenesis of pulmonary fibrosis. In addition to generating collagen deposition, immunization of mice with dually-modified vimentin demonstrated the highest serum PDGF-BB levels compared to either single modification. In contrast, lung homogenates of mice immunized with modified vimentin showed a reduction in PDGF-BB levels compared to native vimentin. These findings suggest that PDGF-BB is either released into the circulation resulting in increased serum levels or consumed/bound by fibroblasts in the lungs of mice immunized with modified vimentin. Thus, PDGF-BB may serve as an important mediator between post-translational modifications of vimentin and fibrotic lung disease (i.e. RA-ILD).

Figure 1. Trichrome staining of lung tissue in the DBA mice immunized with native and modified vimentin. Mice were immunized with saline, native VIM, VIM-MAA, VIM-CIT, and VIM-MAA-CIT.

Figure 2. PDGF levels in serum of DBA mice immunized with native and modified vimentin. Mice were immunized with saline, native VIM, VIM-MAA, VIM-CIT, and VIM-MAA-CIT. The data is represented as a mean PDGF concentration (pg/mL) and reported with SEM. Comparisons made to Saline are illustrated above each bar: ****p<0.0001, *p<0.05, n≥4. Only significant differences between groups are illustrated: ****p<0.0001, *p<0.05.

Figure 3. PDGF levels in lung homogenates of DBA mice immunized with native and modified vimentin. Mice were immunized with saline, native VIM, VIM-MAA, VIM-CIT, and VIM-MAA-CIT. The data is represented as a mean PDGF concentration (pg/mL) and reported with SEM. Comparisons made to Saline are illustrated above each bar: ****p<0.0001, *p<0.05, n≥4.
N. Aripova: None; M. Duryee: None; C. Hunter: None; A. Nelson: None; B. Butler: None; J. Poole: AstraZeneca, 12, I have received no monies. I received anti-IL-33 monoclonal antibody from AstraZeneca for animal research sutdies.; B. England: Boehringer-Ingelheim, 2, 5; G. Thiele: None; T. Mikuls: Elsevier, 9, Horizon Therapeutics, 2, 5, Pfizer, 2, Sanofi, 2, UCB Pharma, 2, Wolters Kluwer Health (UpToDate), 9.



