Poster Session C
Systemic lupus erythematosus (SLE)
Session: (2257–2325) SLE – Diagnosis, Manifestations, & Outcomes Poster III
2302: Persistently Active Disease in Adult Patients with Childhood Onset Systemic Lupus Erythematosus
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- AH
Alberta Hoi, MD, PhD
Monash University
Melbourne, Victoria, AustraliaDisclosure(s): No financial relationships with ineligible companies to disclose
Abstract Poster Presenter(s)
Rachel Koelmeyer1, Kate gregory1, Rangi Kandane-Rathnayake2, Fiona Goldblatt3, Sean O’Neill4, Maureen Rischmueller5, Mandana Nikpour6, Geraldine Hassett7, Pravin Hissaria8, Darakanathan Ranganathan9, Claire Barrett10, Ashleigh Hennessey9, Ted Tsai11, Peter Gowdie12, Eric Morand13 and Alberta Hoi14, 1Monash University, Clayton, Australia, 2Monash University, Department of Medicine, Sub-faculty of Clinical and Molecular Medicine, Clayton, Australia, 3Royal Adelaide Hospital and Flinders Medical Centre, Adelaide, Australia, 4Department of Medicine, University of New South Wales, Kensington, Australia, 5The Queen Elizabeth Hospital and Basil Hetzel Institute; Adelaide Medical School, University of Adelaide, Adelaide, Australia, 6The University of Melbourne at St. Vincent’s Hospital Melbourne, Departments of Medicine and Rheumatology, Melbourne, Australia, 7Liverpool Hospital, Liverpool, Australia, 8Royal Adelaide Hospital, Adelaide, Australia, 9Royal Brisbane and Women's Hospital, Herston, Australia, 10Redcliffe Hospital, Redcliffe, Australia, 11Canberra Hospital, Garran, Australia, 12Monash Health, Clayton, Australia, 13Monash University, Centre for Inflammatory Diseases, Melbourne, Australia, 14Monash University, Department of Medicine, Sub-faculty of Clinical and Molecular Medicine, Melbourne, Australia
Background/Purpose: Previous studies in childhood-onset systemic lupus erythematosus (cSLE) have suggested that cSLE patients have more severe disease, compared to those with adult-onset disease (aSLE). These reported associations are mostly based on cross-sectional observational studies. We compared longitudinal disease characteristics, medication use, and outcomes in cSLE versus aSLE patients in adulthood.
Methods: We followed adult patients who fulfilled ACR or SLICC classification criteria for SLE, enrolled in the Australian Lupus Registry and Biobank (ALRB), a multicentre observational cohort, between 2007 and 2022. Patients who were aged < 18 at diagnosis were grouped as cSLE, and patients aged ≥18years were grouped as aSLE. Patients' disease activity assessed using SLEDAI-2K, SFI and PGA, and medication details were captured at each routine visit while organ damage (using SDI) and HRQoL (using SF36v2) were captured annually. Lupus low disease activity state (LLDAS) and high disease activity status (HDAS, ever SLEDAI-2K score ≥10) are derived as previously published. Triple therapy is defined as concurrent use of immunosuppressant, hydroxychloroquine and corticosteroid. Comparisons were made using appropriate bivariate tests.
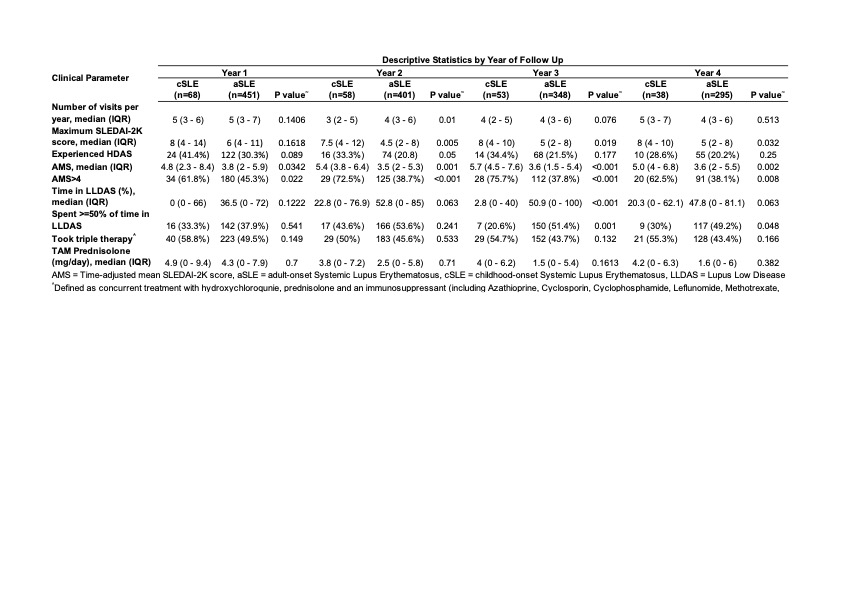
Results: The cohort consisted of 519 patients from 9 ALRB centres, with a median age of diagnosis of 29 [IQR: 22,42] years. 68 patients (13%) had cSLE. Despite their younger age at enrolment (25 vs 41 years, p< 0.001), a higher proportion of patients with cSLE already had damage present (SDI≥1) at enrolment (53% vs 40%, p=0.05) consistent with their longer disease duration (median length of disease for cSLE 13.2 vs aSLE 4.2, p< 0.001). cSLE patients had more renal involvement at enrolment (62% cSLE vs 37% aSLE, p< 0.001). The median (IQR) observation period was 4.8 (2.6, 9.3) years. Disease activity, as measured by enrolment SLEDAI-2K (median (IQR) of 8 (4,12) vs 4 (2,8), p=0.013) and time-adjusted mean SLEDAI (AMS) (median (IQR) of 5.0 (3.0, 6.4) cSLE vs 3.6 (1.9,5.1) aSLE, p< 0.001) were significantly higher in cSLE patients, and the difference persisted in each year of observation (Table 1). A higher proportion of cSLE patients had AMS >4, or had HDAS. Only 36% cSLE vs 51% aSLE (p=0.03) spent ³50% time in LLDAS. The domains of disease activity and visit frequency were similar between the two groups. A high proportion of cSLE patients had been treated with triple therapy at enrolment (50% vs 35.5%, p=0.021), and have used mycophenolate (58.8% vs 38.8%, p=0.002). Exposure to Rituximab was seen in 14.7% cSLE vs 8.7% aSLE (p=0.111) whereas exposure to cyclophosphamide was seen in 8.8% cSLE vs 6.0% aSLE (p=0.371).
Conclusion: cSLE patients had severe disease that was discernible at enrolment and persisted during follow-up. This difference was not due to fewer review visits, disproportionate involvement of certain organ domains, or undertreatment with conventional immunosuppressants. These findings should raise awareness amongst clinicians to consider early aggressive control of disease, including earlier consideration of biologic therapy.
R. Koelmeyer: None; K. gregory: None; R. Kandane-Rathnayake: None; F. Goldblatt: None; S. O’Neill: None; M. Rischmueller: AbbVie/Abbott, 2, 5, 6, Boehringer-Ingelheim, 2, 6, Bristol-Myers Squibb(BMS), 5, Eli Lilly, 5, GlaxoSmithKlein(GSK), 5, Janssen, 5, Novartis, 2, 5, 6, Recordati Rare Diseases, 2, 6, Servier, 5, UCB, 5; M. Nikpour: AstraZeneca, 2, 6, Boehringer-Ingelheim, 2, 6, GSK, 2, 6, Janssen Pharmaceuticals, 2, 5, 6; G. Hassett: None; P. Hissaria: None; D. Ranganathan: None; C. Barrett: None; A. Hennessey: None; T. Tsai: None; P. Gowdie: None; E. Morand: AbbVie, 2, 5, Amgen, 5, AstraZeneca, 2, 5, 6, Biogen, 2, 5, Bristol Myers Squibb, 2, 5, Eli Lilly, 2, 5, EMD Serono, 2, 5, Galapagos, 2, Genentech, 2, 5, GlaxoSmithKline, 2, 5, IgM, 2, Janssen, 2, 5, Novartis, 2, Servier, 2, Takeda, 2, UCB, 5; A. Hoi: Abbvie, 6, AstraZeneca, 5, Australian Rheumatology Association, 4, Eli Lilly, 6, EUSA Pharma (UK) Limited, 2, Limbic, 6, Moose Republic, 6, Novartis, 6.
Background/Purpose: Previous studies in childhood-onset systemic lupus erythematosus (cSLE) have suggested that cSLE patients have more severe disease, compared to those with adult-onset disease (aSLE). These reported associations are mostly based on cross-sectional observational studies. We compared longitudinal disease characteristics, medication use, and outcomes in cSLE versus aSLE patients in adulthood.
Methods: We followed adult patients who fulfilled ACR or SLICC classification criteria for SLE, enrolled in the Australian Lupus Registry and Biobank (ALRB), a multicentre observational cohort, between 2007 and 2022. Patients who were aged < 18 at diagnosis were grouped as cSLE, and patients aged ≥18years were grouped as aSLE. Patients' disease activity assessed using SLEDAI-2K, SFI and PGA, and medication details were captured at each routine visit while organ damage (using SDI) and HRQoL (using SF36v2) were captured annually. Lupus low disease activity state (LLDAS) and high disease activity status (HDAS, ever SLEDAI-2K score ≥10) are derived as previously published. Triple therapy is defined as concurrent use of immunosuppressant, hydroxychloroquine and corticosteroid. Comparisons were made using appropriate bivariate tests.
Results: The cohort consisted of 519 patients from 9 ALRB centres, with a median age of diagnosis of 29 [IQR: 22,42] years. 68 patients (13%) had cSLE. Despite their younger age at enrolment (25 vs 41 years, p< 0.001), a higher proportion of patients with cSLE already had damage present (SDI≥1) at enrolment (53% vs 40%, p=0.05) consistent with their longer disease duration (median length of disease for cSLE 13.2 vs aSLE 4.2, p< 0.001). cSLE patients had more renal involvement at enrolment (62% cSLE vs 37% aSLE, p< 0.001). The median (IQR) observation period was 4.8 (2.6, 9.3) years. Disease activity, as measured by enrolment SLEDAI-2K (median (IQR) of 8 (4,12) vs 4 (2,8), p=0.013) and time-adjusted mean SLEDAI (AMS) (median (IQR) of 5.0 (3.0, 6.4) cSLE vs 3.6 (1.9,5.1) aSLE, p< 0.001) were significantly higher in cSLE patients, and the difference persisted in each year of observation (Table 1). A higher proportion of cSLE patients had AMS >4, or had HDAS. Only 36% cSLE vs 51% aSLE (p=0.03) spent ³50% time in LLDAS. The domains of disease activity and visit frequency were similar between the two groups. A high proportion of cSLE patients had been treated with triple therapy at enrolment (50% vs 35.5%, p=0.021), and have used mycophenolate (58.8% vs 38.8%, p=0.002). Exposure to Rituximab was seen in 14.7% cSLE vs 8.7% aSLE (p=0.111) whereas exposure to cyclophosphamide was seen in 8.8% cSLE vs 6.0% aSLE (p=0.371).
Conclusion: cSLE patients had severe disease that was discernible at enrolment and persisted during follow-up. This difference was not due to fewer review visits, disproportionate involvement of certain organ domains, or undertreatment with conventional immunosuppressants. These findings should raise awareness amongst clinicians to consider early aggressive control of disease, including earlier consideration of biologic therapy.

Table 1. Pattern of visit frequency, disease activity, and medication use in each year of followup between cSLE vs aSLE patients.
R. Koelmeyer: None; K. gregory: None; R. Kandane-Rathnayake: None; F. Goldblatt: None; S. O’Neill: None; M. Rischmueller: AbbVie/Abbott, 2, 5, 6, Boehringer-Ingelheim, 2, 6, Bristol-Myers Squibb(BMS), 5, Eli Lilly, 5, GlaxoSmithKlein(GSK), 5, Janssen, 5, Novartis, 2, 5, 6, Recordati Rare Diseases, 2, 6, Servier, 5, UCB, 5; M. Nikpour: AstraZeneca, 2, 6, Boehringer-Ingelheim, 2, 6, GSK, 2, 6, Janssen Pharmaceuticals, 2, 5, 6; G. Hassett: None; P. Hissaria: None; D. Ranganathan: None; C. Barrett: None; A. Hennessey: None; T. Tsai: None; P. Gowdie: None; E. Morand: AbbVie, 2, 5, Amgen, 5, AstraZeneca, 2, 5, 6, Biogen, 2, 5, Bristol Myers Squibb, 2, 5, Eli Lilly, 2, 5, EMD Serono, 2, 5, Galapagos, 2, Genentech, 2, 5, GlaxoSmithKline, 2, 5, IgM, 2, Janssen, 2, 5, Novartis, 2, Servier, 2, Takeda, 2, UCB, 5; A. Hoi: Abbvie, 6, AstraZeneca, 5, Australian Rheumatology Association, 4, Eli Lilly, 6, EUSA Pharma (UK) Limited, 2, Limbic, 6, Moose Republic, 6, Novartis, 6.



