Poster Session C
Rheumatoid arthritis (RA)
Session: (2095–2140) RA – Diagnosis, Manifestations, and Outcomes Poster III
2134: Autoantibodies to Joint-related Proteins Predict Remission with High Specificity in New Onset RA
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- IG
Inger Gjertsson, MD, PhD (she/her/hers)
Institutionen för medicin, avd för reumatologi och inflammationsforskning
Goteborg, SwedenDisclosure information not submitted.
Abstract Poster Presenter(s)
Monica Leu Agelii1, Outi Sareila2, Erik Lönnblom2, Kristina Forslind3, Maria Andersson3, Alf Kastbom4, Christopher Sjöwall5, Lennart Jacobsson6, Jan Kihlberg7, Rikard Holmdahl2, Ingiäld Hafström2 and Inger Gjertsson8, 1Gothenburg University, Goteborg, Sweden, 2Karolinska Institutet, Stockholm, Sweden, 3FoU Spenshult, Halmstad, Sweden, 4Linköping University, Linköping, Sweden, 5Department of Biomedical and Clinical Sciences, Division of Inflammation and Infection/Rheumatology, Linköping University, Linköping, Sweden, 6University of Gothenburg, Malmö, Sweden, 7Uppsala University, Uppsala, Sweden, 8Department of Rheumatology and Inflammation research, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
Background/Purpose: One of the major challenges in the management of rheumatoid arthritis (RA) is to determine individual treatment and predict the prognosis. On a group level, high disease activity and antibodies specific for citrulline (ACPA) and immunoglobulins (RF) are factors associated with poor prognosis. New alternative biomarkers, which could provide more specific information, are antibodies to disease-related targets in the joints.
The purpose of this study is to identify circulating autoantibodies to joint-related proteins (hereafter named JointIDs) that predict disease outcome in patients with new onset RA
Methods: Sera at diagnosis from BARFOT and TIRA-2 cohorts (n=1986) with new onset RA patients were screened with a bead-based multiplex flow immunoassay to detect IgG autoantibodies against 47 peptides derived from joint proteins (JointIDs). Disease outcomes included Boolean remission at 6 and 12 months, swollen joint count (SJC) and radiological progression at 12 months (>8% affected feet joints and >3% affected hand joints) in patients who were without joint damage at inclusion. Multivariate logistic regression and zero-inflated negative binomial models adjusted for clinical factors (age, gender, ACPA, RF, changes in medication at 3 and 6 months) were used to identify JointIDs with the strongest potential to predict prognosis
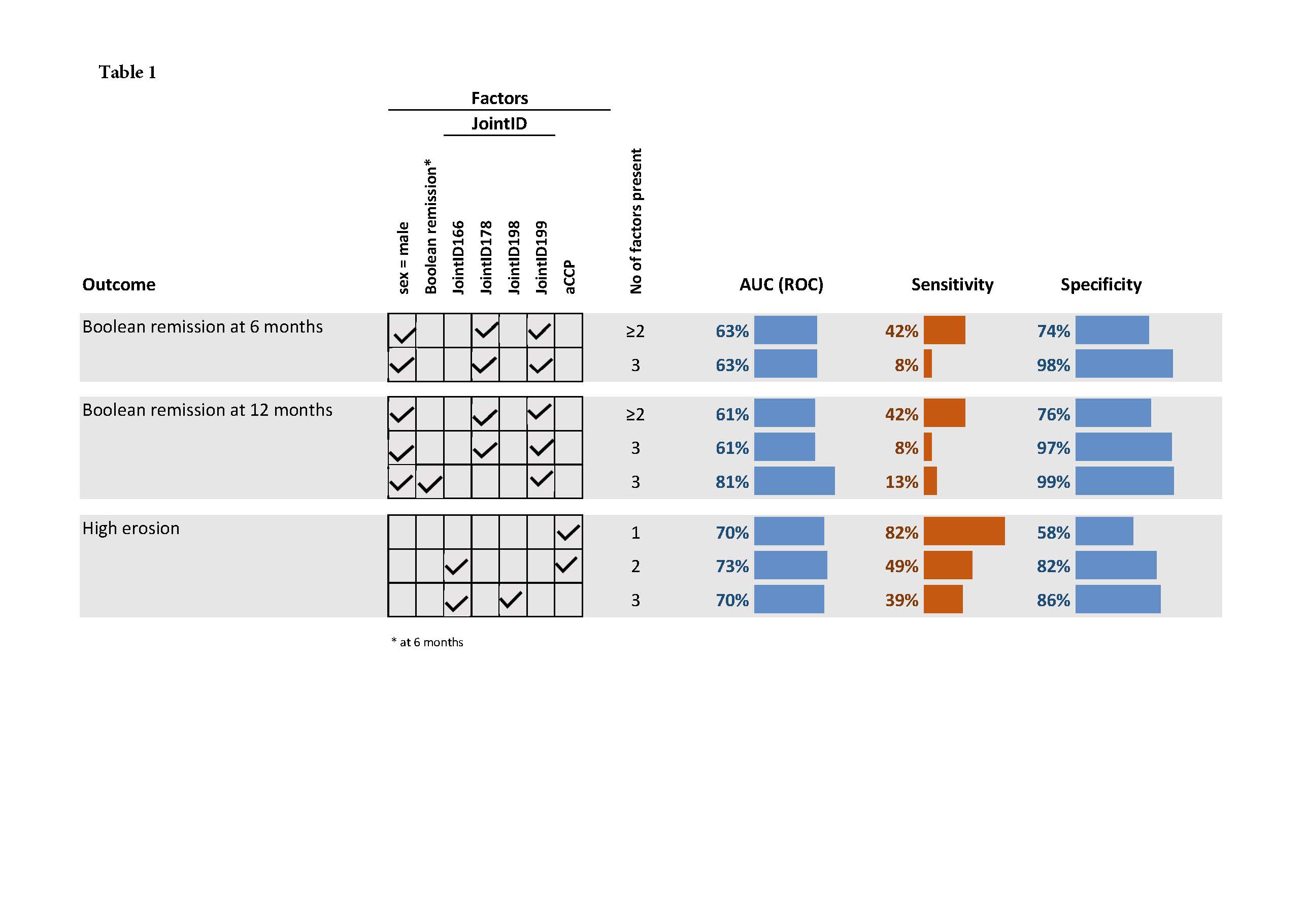
Results: Six JointIDs were identified as predictors for these disease outcomes in multivariate analyses. Presence of JointID178 predicted an average decrease in SJC at 6 months with 41% and with 33% at 12 months. Presence of at least 2 of the following factors: JointID178 positive and JointID199 negative at inclusion in combination with male sex identified approx. 40% of the patients in Boolean remission at 6 months with approx. 75% specificity (Table 1). A similar test performance was obtained even for Disease Activity Score 28 remission. The sensitivity and specificity for being in Boolean remission at 12 months if the patients were male, negative for JointID199 and in Boolean remission at 6 months was 13% (CI 9%;16%) and 99% (CI 99%;100%), respectively. RF and ACPA did not predict remission but were the strongest predictors of joint destruction at 12 months although with low specificity (58%). Addition of JointID166 significantly increased the specificity at the expense of sensitivity.
Conclusion: Autoantibodies to joint-related proteins at RA diagnosis can predict remission with a high specificity at 6 and 12 months. This knowledge is of clinical importance as patients positive or negative for these JointIDs in combination with clinical factors may potentially guide future treatment choices.
M. Leu Agelii: None; O. Sareila: Vacara AB, 3; E. Lönnblom: Vacara AB, 2; K. Forslind: None; M. Andersson: None; A. Kastbom: None; C. Sjöwall: None; L. Jacobsson: Novartis, Eli-Lily and Janssen, 6, Pfizer, Novartis, Eli-Lily and Janssen., 1; J. Kihlberg: None; R. Holmdahl: Regor, Lipum AB and Cyxone AB,, 2; I. Hafström: None; I. Gjertsson: None.
Background/Purpose: One of the major challenges in the management of rheumatoid arthritis (RA) is to determine individual treatment and predict the prognosis. On a group level, high disease activity and antibodies specific for citrulline (ACPA) and immunoglobulins (RF) are factors associated with poor prognosis. New alternative biomarkers, which could provide more specific information, are antibodies to disease-related targets in the joints.
The purpose of this study is to identify circulating autoantibodies to joint-related proteins (hereafter named JointIDs) that predict disease outcome in patients with new onset RA
Methods: Sera at diagnosis from BARFOT and TIRA-2 cohorts (n=1986) with new onset RA patients were screened with a bead-based multiplex flow immunoassay to detect IgG autoantibodies against 47 peptides derived from joint proteins (JointIDs). Disease outcomes included Boolean remission at 6 and 12 months, swollen joint count (SJC) and radiological progression at 12 months (>8% affected feet joints and >3% affected hand joints) in patients who were without joint damage at inclusion. Multivariate logistic regression and zero-inflated negative binomial models adjusted for clinical factors (age, gender, ACPA, RF, changes in medication at 3 and 6 months) were used to identify JointIDs with the strongest potential to predict prognosis
Results: Six JointIDs were identified as predictors for these disease outcomes in multivariate analyses. Presence of JointID178 predicted an average decrease in SJC at 6 months with 41% and with 33% at 12 months. Presence of at least 2 of the following factors: JointID178 positive and JointID199 negative at inclusion in combination with male sex identified approx. 40% of the patients in Boolean remission at 6 months with approx. 75% specificity (Table 1). A similar test performance was obtained even for Disease Activity Score 28 remission. The sensitivity and specificity for being in Boolean remission at 12 months if the patients were male, negative for JointID199 and in Boolean remission at 6 months was 13% (CI 9%;16%) and 99% (CI 99%;100%), respectively. RF and ACPA did not predict remission but were the strongest predictors of joint destruction at 12 months although with low specificity (58%). Addition of JointID166 significantly increased the specificity at the expense of sensitivity.
Conclusion: Autoantibodies to joint-related proteins at RA diagnosis can predict remission with a high specificity at 6 and 12 months. This knowledge is of clinical importance as patients positive or negative for these JointIDs in combination with clinical factors may potentially guide future treatment choices.

M. Leu Agelii: None; O. Sareila: Vacara AB, 3; E. Lönnblom: Vacara AB, 2; K. Forslind: None; M. Andersson: None; A. Kastbom: None; C. Sjöwall: None; L. Jacobsson: Novartis, Eli-Lily and Janssen, 6, Pfizer, Novartis, Eli-Lily and Janssen., 1; J. Kihlberg: None; R. Holmdahl: Regor, Lipum AB and Cyxone AB,, 2; I. Hafström: None; I. Gjertsson: None.



