Poster Session C
Rheumatoid arthritis (RA)
Session: (1734–1775) RA – Etiology and Pathogenesis Poster
1771: Role of Periostin in the Pathophysiology and Accurate Diagnosis of Interstitial Lung Disease Associated with Autoimmune Diseases
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- VP
Abstract Poster Presenter(s)
Verónica Pulito-Cueto1, David Iturbe-Fernández2, Victor Mora-Cuesta2, Joao Carlos Batista-Liz1, Belén Atienza-Mateo3, María Sebastián-Mora1, Virginia Portilla1, Alfonso Corrales1, Miguel A Gonzalez-Gay4, Jose Cifrian5, Ricardo Blanco1 and Raquel López-Mejías1, 1Rheumatology Department, Immunopathology Group, Hospital Universitario Marqués de Valdecilla-IDIVAL, Santander, Spain, 2Immunopathology Group, Marqués de Valdecilla University Hospital-IDIVAL; Department of Pneumology, Marqués de Valdecilla University Hospital, Santander, Spain, 3Rheumatology, Hospital Universitario Marqués de Valdecilla, IDIVAL, Santander, Spain, 4IDIVAL and School of Medicine, UC, Santander; Department of Rheumatology, IIS-Fundación Jiménez Díaz, Madrid, Santander, Spain, 5Immunopathology Group, Marqués de Valdecilla University Hospital-IDIVAL; Department of Pneumology, Marqués de Valdecilla University Hospital; School of Medicine, Cantabria University, Santander, Spain
Background/Purpose: Periostin is an extracellular matrix protein that contributes to the development and repair of lung tissue [1]. Previous works have described periostin as a key factor in the aberrant evolution of the airways and parenchymal fibrosis, being involved in the pathophysiology of different chronic lung diseases such as interstitial lung diseases (ILD) [1-3]. In this sense, ILD is one of the most frequent manifestations and the main cause of death in patients with autoimmune disease (AD), particularly in rheumatoid arthritis (RA), systemic sclerosis (SSc), inflammatory myopathies (IM) and Sjogren's syndrome (SS) [4]. However, the accurate diagnosis of AD-ILD+ often remains a challenge due partially to the similarity with other diseases such as idiopathic pulmonary fibrosis (IPF) and interstitial pneumonia with autoimmune features (IPAF) [4].Accordingly, this work aimed to elucidate the role of periostin in the accurate diagnosis of AD-ILD+, as well as its relationship with molecules involved in the pathological process of vasculopathy and fibrosis typical of AD-ILD+.
Methods: Peripheral venous blood was collected from a total of 98 patients with AD-ILD+, a group composed of patients with RA‐ILD+ (n=33), SSc‐ILD+ (n=38), IM- ILD+ (n=23) and SS-ILD+ (n=4). Moreover, we recruited different comparative groups: 105 IPF patients, 11 IPAF patients and 43 healthy controls (HC). Serum levels of periostin were measured by enzyme-linked immunosorbent assay (ELISA). Additionally, serum levels of Krebs von den Lungen 6 (KL-6); interleukin 6 (IL-6); endothelin-1 (ET-1); vascular endothelial growth factor (VEGF) and intercellular adhesion molecule 1 (ICAM-1) were measured by ELISA.
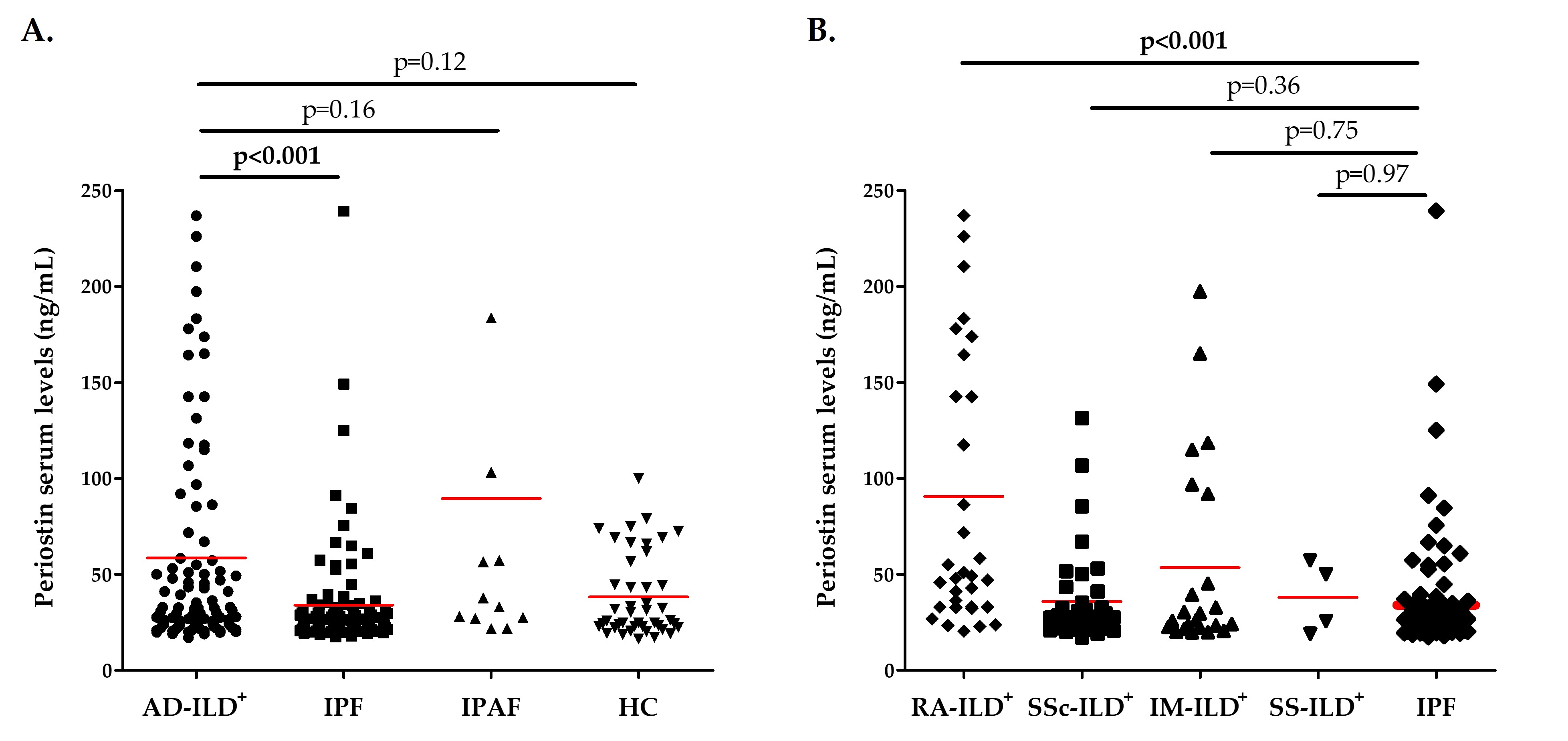
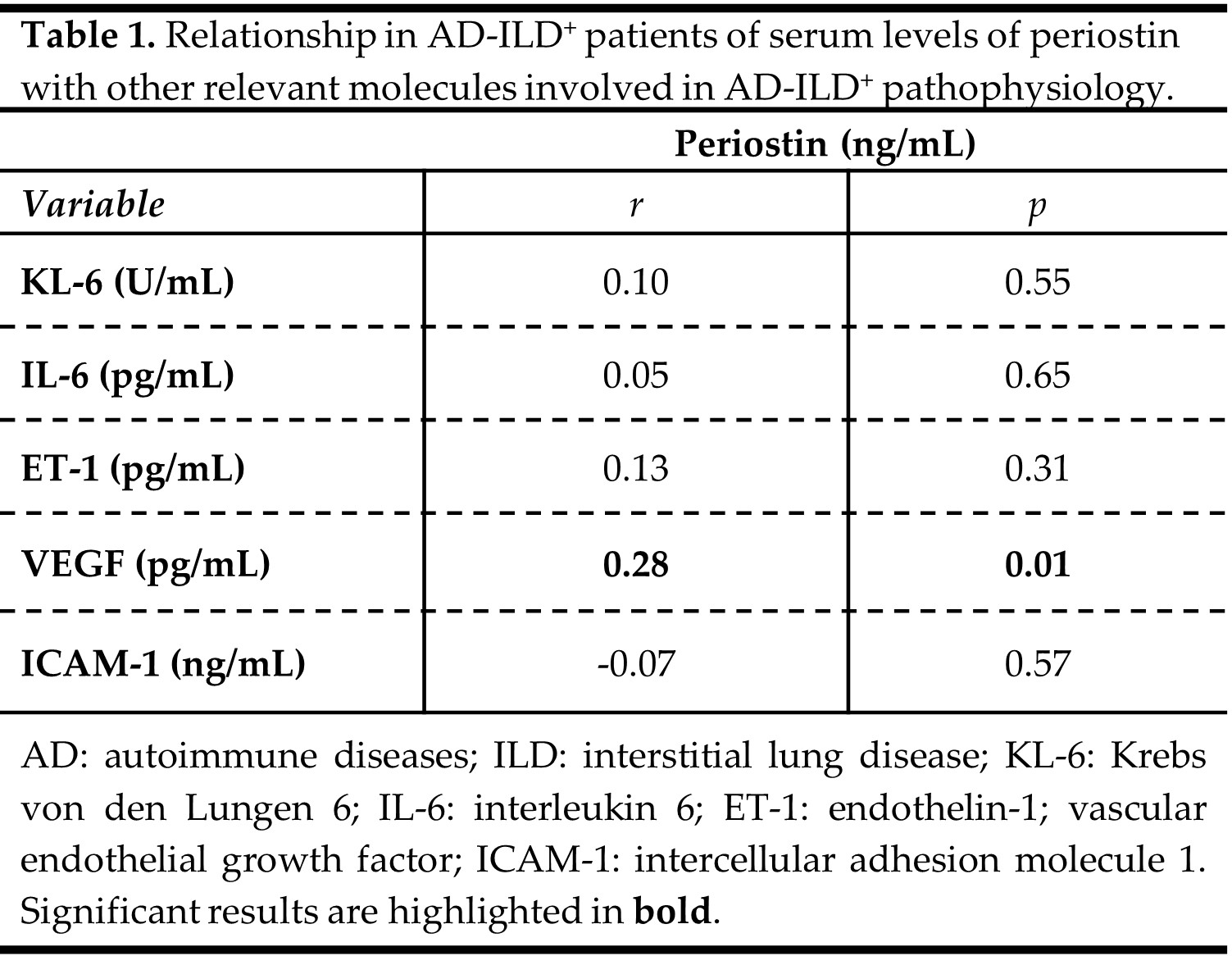
Results: Patients with AD-ILD+ exhibited significantly higher periostin serum levels than IPF patients (p< 0.001, Figure 1A), whereas no difference was observed in relation to IPAF patients and HC (Figure 1A). Specifically, RA-ILD+ patients showed increased levels of periostin in relation to IPF patients (p< 0.001, Figure 1B). However, similar levels of periostin were found in patients with SSc-ILD+, IM-ILD+ as well as SS-ILD+ and those with IPF (Figure 1B). Furthermore, a positive correlation was discovered between periostin serum levels and VEGF serum concentrations in AD-ILD+ patients (p=0.01, Table 1). Nevertheless, periostin serum levels did not correlate with KL-6, IL-6, ET-1 and ICAM-1 levels in AD-ILD+ patients (Table 1).
Conclusion: Our findings suggest that circulating periostin could be considered a useful biomarker to discriminate RA-ILD+ from IPF, contributing to the accurate diagnosis of RA-ILD+. Furthermore, this work indicates a relationship of periostin with vascular dysfunction in AD-ILD+. References: [1] Cell Mol Life Sci.2017;74(23):4305-4314; [2] Respir Investig.2015;53(2):73-81; [3] PLoS One.2017;12(3):e0174547; [4] Expert Rev Clin Immunol.2018;14(1):69-82. Personal funds, VP-C: PI18/00042(ISCIII-ERDF); JB-L:FI22/00020(ISCIII-ESF); MSM-G:TRANSVAL22/01(IDIVAL); RL-M: CPII21/00004(ISCIII-ESF).
V. Pulito-Cueto: None; D. Iturbe-Fernández: None; V. Mora-Cuesta: None; J. Batista-Liz: None; B. Atienza-Mateo: None; M. Sebastián-Mora: None; V. Portilla: None; A. Corrales: None; M. Gonzalez-Gay: AbbVie/Abbott, 5, 6, Amgen, 5, 6, Pfizer, 5, 6; J. Cifrian: None; R. Blanco: AbbVie/Abbott, 5, 6, Amgen, 6, AstraZeneca, 2, Bristol-Myers Squibb(BMS), 6, Eli Lilly, 6, Galapagos, 2, 6, Janssen, 2, 6, Merck/MSD, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche, 5, 6, Sanofi, 6; R. López-Mejías: None.
Background/Purpose: Periostin is an extracellular matrix protein that contributes to the development and repair of lung tissue [1]. Previous works have described periostin as a key factor in the aberrant evolution of the airways and parenchymal fibrosis, being involved in the pathophysiology of different chronic lung diseases such as interstitial lung diseases (ILD) [1-3]. In this sense, ILD is one of the most frequent manifestations and the main cause of death in patients with autoimmune disease (AD), particularly in rheumatoid arthritis (RA), systemic sclerosis (SSc), inflammatory myopathies (IM) and Sjogren's syndrome (SS) [4]. However, the accurate diagnosis of AD-ILD+ often remains a challenge due partially to the similarity with other diseases such as idiopathic pulmonary fibrosis (IPF) and interstitial pneumonia with autoimmune features (IPAF) [4].Accordingly, this work aimed to elucidate the role of periostin in the accurate diagnosis of AD-ILD+, as well as its relationship with molecules involved in the pathological process of vasculopathy and fibrosis typical of AD-ILD+.
Methods: Peripheral venous blood was collected from a total of 98 patients with AD-ILD+, a group composed of patients with RA‐ILD+ (n=33), SSc‐ILD+ (n=38), IM- ILD+ (n=23) and SS-ILD+ (n=4). Moreover, we recruited different comparative groups: 105 IPF patients, 11 IPAF patients and 43 healthy controls (HC). Serum levels of periostin were measured by enzyme-linked immunosorbent assay (ELISA). Additionally, serum levels of Krebs von den Lungen 6 (KL-6); interleukin 6 (IL-6); endothelin-1 (ET-1); vascular endothelial growth factor (VEGF) and intercellular adhesion molecule 1 (ICAM-1) were measured by ELISA.
Results: Patients with AD-ILD+ exhibited significantly higher periostin serum levels than IPF patients (p< 0.001, Figure 1A), whereas no difference was observed in relation to IPAF patients and HC (Figure 1A). Specifically, RA-ILD+ patients showed increased levels of periostin in relation to IPF patients (p< 0.001, Figure 1B). However, similar levels of periostin were found in patients with SSc-ILD+, IM-ILD+ as well as SS-ILD+ and those with IPF (Figure 1B). Furthermore, a positive correlation was discovered between periostin serum levels and VEGF serum concentrations in AD-ILD+ patients (p=0.01, Table 1). Nevertheless, periostin serum levels did not correlate with KL-6, IL-6, ET-1 and ICAM-1 levels in AD-ILD+ patients (Table 1).
Conclusion: Our findings suggest that circulating periostin could be considered a useful biomarker to discriminate RA-ILD+ from IPF, contributing to the accurate diagnosis of RA-ILD+. Furthermore, this work indicates a relationship of periostin with vascular dysfunction in AD-ILD+. References: [1] Cell Mol Life Sci.2017;74(23):4305-4314; [2] Respir Investig.2015;53(2):73-81; [3] PLoS One.2017;12(3):e0174547; [4] Expert Rev Clin Immunol.2018;14(1):69-82. Personal funds, VP-C: PI18/00042(ISCIII-ERDF); JB-L:FI22/00020(ISCIII-ESF); MSM-G:TRANSVAL22/01(IDIVAL); RL-M: CPII21/00004(ISCIII-ESF).

Figure 1. Role of periostin in the accurate diagnosis of AD-ILD+. A. Differences in periostin serum levels between patients with AD-ILD+ and those with IPF and IPAF, as well as HC; B. Differences in periostin serum levels between patients with AD-ILD+ stratified by the underlying AD and those with IPF. AD: autoimmune diseases; ILD: interstitial lung disease; IPF: idiopathic pulmonary fibrosis; IPAF: interstitial pneumonia with autoimmune features; HC: healthy controls; RA: rheumatoid arthritis; SSc: systemic sclerosis; IM: inflammatory myopathies; SS: sjögren syndrome. Significant results are highlighted in bold.

V. Pulito-Cueto: None; D. Iturbe-Fernández: None; V. Mora-Cuesta: None; J. Batista-Liz: None; B. Atienza-Mateo: None; M. Sebastián-Mora: None; V. Portilla: None; A. Corrales: None; M. Gonzalez-Gay: AbbVie/Abbott, 5, 6, Amgen, 5, 6, Pfizer, 5, 6; J. Cifrian: None; R. Blanco: AbbVie/Abbott, 5, 6, Amgen, 6, AstraZeneca, 2, Bristol-Myers Squibb(BMS), 6, Eli Lilly, 6, Galapagos, 2, 6, Janssen, 2, 6, Merck/MSD, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche, 5, 6, Sanofi, 6; R. López-Mejías: None.



