Poster Session C
Rheumatoid arthritis (RA)
Session: (2141–2176) RA – Treatment Poster III
2174: JAK Inhibitors in Rheumatoid Arthritis-Interstitial Lung Disease. National Multicenter Study of 73 Patients, 55 of Baricitinib
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- AS
Ana Serrano-Combarro, MD
Hospital Universitario Marqués de Valdecilla, IDIVAL
Santander, SpainDisclosure information not submitted.
Abstract Poster Presenter(s)
Ana Serrano-Combarro1, Belén Atienza-Mateo2, Jesus Alejandro Valero-Jaimes3, Marta Pastor-Mena4, Rafael Benito Melero-Gonzalez5, David Castro-Corredor6, Maria Martin-Lopez7, Santos Castañeda8, Jesus Loarce-Martos9, Natalia Mena Vazquez10, Carmen carrasco-Cubero11, Carolina Diez-Morrondo12, andrea Garcia-Valle13, Gema Bonilla14, Juan Maria Blanco-Madrigal15, Natividad del Val del Amo16, Nuria Vegas Revenga17, Lorena Perez-Albadalejo18, Rafaela Ortega Castro19, Deseada Palma-Sanchez20, Ana Maria fernandez-Ortiz21, Patricia Lopez-Viejo22, Maria Lopez-Lasanta23, Marta Garijo Bufort24, Ivette Casafont-Sole25, Olga Maiz-Alonso26, Juan Moreno-Morales27, Ana Urruticoechea28, Carolina Perez-Garcia29, Jose Rosas30, Virginia Ruiz-Esquide31, Delia Fernández-Lozano32, Ignacio Brana Abascal33, Evelin Cecilia Cervantes-Perez34, Julia Fernandez-Melon35, Cristina Fernandez36, Bryan Josue Flores Robles37, Diego Ferrer38 and Ricardo Blanco39, 1Hospital Universitario Marques de Valdecilla, IDIVAL, Santander, Spain, 2Rheumatology, Hospital Universitario Marqués de Valdecilla, IDIVAL, Santander, Spain, 3Hospital Bidasoa, Irún, Spain, 4Hospital de Jerez de la Frontera, Cádiz, Spain, 5CHU Vigo, O Carballino, Spain, 6General University Hospital of Ciudad Real, Santa Cruz de Mudela (Ciudad Real), Spain, 7Hospital 12 de Octubre, Madrid, Spain, 8Hospital Universitario de la Princesa, Madrid, Spain, 9Ramón y Cajal University Hospital, Madrid, Spain, 10IBIMA, Málaga, Spain, 11Department of Rheumatology, Hospital Universitario de Badajoz, Badajoz, Spain, 12Division of Rheumatology, Hospital de León, León, Spain, 13Division of Rheumatology, Complejo Asistencial Universitario de Palencia, Palencia, Spain, 14Department of Rheumatology, Hospital Clínico Universitario La Paz, Madrid, Spain, 15Division of Rheumatology, H. Universitario de Basurto, Bilbao, Spain, 16Complejo Hospitalario de Navarra, Pamplona, Spain, 17Hospital Galdakao- Usansolo, Galdakao, Spain, 18Division of Rheumatology, Hospital Universitario de Jaén, Jaén, Spain, 19Hospital Reina Sofía, Cordoba, Spain, 20Hospital Rafael Mendez, Lorca, Spain, 21CHU Badajoz, Badajoz, Spain, 22Division of Rheumatology, Hospital Severo Ochoa, Leganéz, Spain, 23Hospital Universitari Vall d'Hebron, Rheumatology, Barcelona, Spain, 24H. de Sagunto, Valencia, Italy, 25Hospital Universitari Germans Trias i Pujol, Badalona, Spain, 26University Hospital Donostia, San Sebastian, Spain, 27Hospital Universitario Santa Lucia Cartagena, Cartagena, Spain, 28Hospital Can Misses, Ibiza, Spain, 29Hospital del Mar, Barcelona, Spain, 30Hospital Marina Baixa, Alicante, Spain, 31Hospital Clinic, Rheumatology, Barcelona, Spain, 32Hospital de Mérida, Merida, Spain, 33Division of Rheumatology, Hospital Universitario Central de Asturias, Oviedo, Spain, 34Complexo Hospitalario Universitario de Pontevedra, Pontevedra, Spain, 35Division of Rheumatology, Hospital Universitario Son Espases, Palma de Mallorca, Spain, 36Hospital Universitario San Juan de Alicante, Alicante, Spain, 37Hospital Universitario San Pedro, Logroño, Spain, 38Division of Pneumology, Hospital Universitario Marqués de Valdecilla, IDIVAL, Santander, Spain, 39Hospital Universitario Marqués de Valdecilla, IDIVAL, Santander, Spain
Background/Purpose: Interstitial lung disease (ILD) is a severe extra-articular manifestation of rheumatoid arthritis (RA). Abatacept and Rituximab are the recommended drugs. JAK inhibitors (JAKi) have demonstrated efficacy in RA. However, in clinical trials patients with active ILD were usually excluded. Moreover, a warning on ILD toxicity is included in SmPC (Summary of Product Characteristics) with tofacitinib (TOFA). Nonetheless, evidence on efficacy of JAKi in RA-ILD is growing. The objective of the study was to assess a) the effectiveness and b) the safety of JAKi in AR-ILD patients.
Methods: National multicenter study of 73 RA-ILD patients on treatment with JAKi. We analyzed from baseline the following outcomes: a) forced vital capacity (FVC), b) diffusing capacity of the lungs for carbon monoxide (DLCO), c) chest high resolution computed tomography (HRCT), d)dyspnea (modified Medical Research Council scale), e) arthritis activity (DAS28-ESR or clinical records), and f) sparing corticosteroids effect.
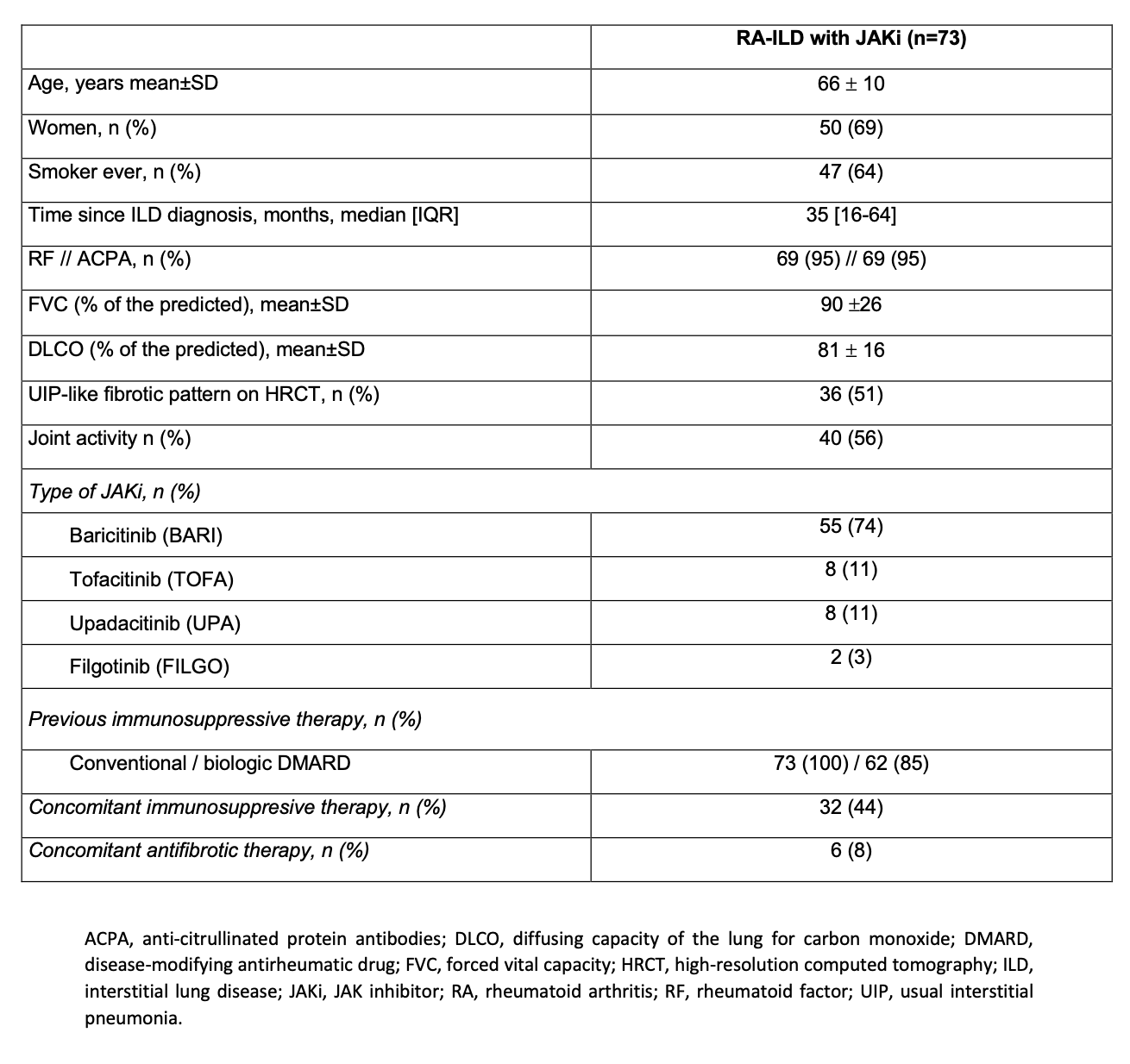
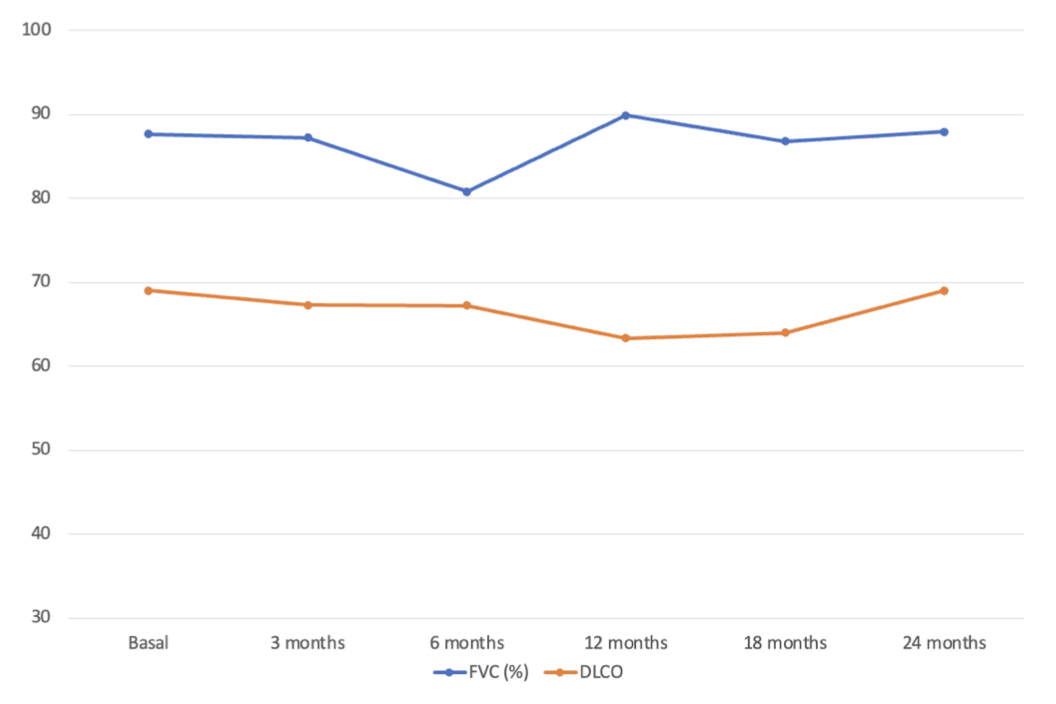
Results: We studied 73 patients (50 women/ 23 men; mean age 66 ± 10 years) from clinical practice on treatment with JAKi [Baricitinib (BARI)= 55 (74%), TOFA= 8 (11%), Upadacitinib (UPA)= 8 (11%), Filgotinib (FILGO)= 2 (3%)]. Baseline demographic and clinical characteristics are shown in Table 1. All patients had received disease-modifying antirheumatic drugs (DMARDs) before JAKi [Methotrexate (63; 86%), Leflunomide (46; 63%), Sulfasalazine (19; 26%), Hydroxychloroquine (16; 22%), Abatacept (47; 64%), Tocilizumab (26; 36%) and Rituximab (16; 22%)]. Since most patients were on BARI we focused on this group (n=55). Median [IQR] ILD duration up to BARI initiation was of 29 [15-64] months. Mean baseline values of FVC and DLCO (% predicted) were 88±27 and 69±20, respectively. Patients were followed-up for a mean of 36 ± 23 months. The evolution of FVC and DLCO remained stable during the first 12 months (Figure 1). At the end of the follow-up, available chest HRCT images improved/ stabilized in 76% of patients. Stabilization or improvement of dyspnea was found in 95% of patients. Most patients showed articular remission or low activity. BARI was withdrawn in 22 (42%) patients due to articular inefficacy (n=15), lung inefficacy (n=4), development of hypersensitivity pneumonitis (n=1), and appearance of brain cancer (n=1).
Conclusion: JAKi, especially BARI, may be useful and safe in controlling the course of both pulmonary and joint disease in RA-ILD patients, even in refractory cases.
A. Serrano-Combarro: None; B. Atienza-Mateo: None; J. Valero-Jaimes: None; M. Pastor-Mena: None; R. Melero-Gonzalez: None; D. Castro-Corredor: None; M. Martin-Lopez: None; S. Castañeda: None; J. Loarce-Martos: Boehringer-Ingelheim, 6, Bristol-Myers Squibb(BMS), 6, Galapagos, 6; N. Mena Vazquez: None; C. carrasco-Cubero: None; C. Diez-Morrondo: None; a. Garcia-Valle: None; G. Bonilla: None; J. Blanco-Madrigal: None; N. del Val del Amo: None; N. Vegas Revenga: None; L. Perez-Albadalejo: None; R. Ortega Castro: None; D. Palma-Sanchez: None; A. fernandez-Ortiz: None; P. Lopez-Viejo: None; M. Lopez-Lasanta: None; M. Garijo Bufort: None; I. Casafont-Sole: None; O. Maiz-Alonso: None; J. Moreno-Morales: None; A. Urruticoechea: None; C. Perez-Garcia: None; J. Rosas: None; V. Ruiz-Esquide: None; D. Fernández-Lozano: None; I. Brana Abascal: None; E. Cervantes-Perez: None; J. Fernandez-Melon: None; C. Fernandez: None; B. Flores Robles: None; D. Ferrer: None; R. Blanco: AbbVie, 5, 6, Amgen, 6, AstraZeneca, 2, BMS, 6, Eli Lilly, 6, Galapagos, 2, 6, Janssen, 2, 6, MSD, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche, 5, 6, Sanofi, 6.
Background/Purpose: Interstitial lung disease (ILD) is a severe extra-articular manifestation of rheumatoid arthritis (RA). Abatacept and Rituximab are the recommended drugs. JAK inhibitors (JAKi) have demonstrated efficacy in RA. However, in clinical trials patients with active ILD were usually excluded. Moreover, a warning on ILD toxicity is included in SmPC (Summary of Product Characteristics) with tofacitinib (TOFA). Nonetheless, evidence on efficacy of JAKi in RA-ILD is growing. The objective of the study was to assess a) the effectiveness and b) the safety of JAKi in AR-ILD patients.
Methods: National multicenter study of 73 RA-ILD patients on treatment with JAKi. We analyzed from baseline the following outcomes: a) forced vital capacity (FVC), b) diffusing capacity of the lungs for carbon monoxide (DLCO), c) chest high resolution computed tomography (HRCT), d)dyspnea (modified Medical Research Council scale), e) arthritis activity (DAS28-ESR or clinical records), and f) sparing corticosteroids effect.
Results: We studied 73 patients (50 women/ 23 men; mean age 66 ± 10 years) from clinical practice on treatment with JAKi [Baricitinib (BARI)= 55 (74%), TOFA= 8 (11%), Upadacitinib (UPA)= 8 (11%), Filgotinib (FILGO)= 2 (3%)]. Baseline demographic and clinical characteristics are shown in Table 1. All patients had received disease-modifying antirheumatic drugs (DMARDs) before JAKi [Methotrexate (63; 86%), Leflunomide (46; 63%), Sulfasalazine (19; 26%), Hydroxychloroquine (16; 22%), Abatacept (47; 64%), Tocilizumab (26; 36%) and Rituximab (16; 22%)]. Since most patients were on BARI we focused on this group (n=55). Median [IQR] ILD duration up to BARI initiation was of 29 [15-64] months. Mean baseline values of FVC and DLCO (% predicted) were 88±27 and 69±20, respectively. Patients were followed-up for a mean of 36 ± 23 months. The evolution of FVC and DLCO remained stable during the first 12 months (Figure 1). At the end of the follow-up, available chest HRCT images improved/ stabilized in 76% of patients. Stabilization or improvement of dyspnea was found in 95% of patients. Most patients showed articular remission or low activity. BARI was withdrawn in 22 (42%) patients due to articular inefficacy (n=15), lung inefficacy (n=4), development of hypersensitivity pneumonitis (n=1), and appearance of brain cancer (n=1).
Conclusion: JAKi, especially BARI, may be useful and safe in controlling the course of both pulmonary and joint disease in RA-ILD patients, even in refractory cases.

Table 1. Baseline characteristics of RA-ILD patients treated with JAKi.

Figure 1. Evolution of pulmonary function tests (mean % of the predicted FVC and DLCO) in RA-ILD patients with BARI therapy at baseline and 24 months.
A. Serrano-Combarro: None; B. Atienza-Mateo: None; J. Valero-Jaimes: None; M. Pastor-Mena: None; R. Melero-Gonzalez: None; D. Castro-Corredor: None; M. Martin-Lopez: None; S. Castañeda: None; J. Loarce-Martos: Boehringer-Ingelheim, 6, Bristol-Myers Squibb(BMS), 6, Galapagos, 6; N. Mena Vazquez: None; C. carrasco-Cubero: None; C. Diez-Morrondo: None; a. Garcia-Valle: None; G. Bonilla: None; J. Blanco-Madrigal: None; N. del Val del Amo: None; N. Vegas Revenga: None; L. Perez-Albadalejo: None; R. Ortega Castro: None; D. Palma-Sanchez: None; A. fernandez-Ortiz: None; P. Lopez-Viejo: None; M. Lopez-Lasanta: None; M. Garijo Bufort: None; I. Casafont-Sole: None; O. Maiz-Alonso: None; J. Moreno-Morales: None; A. Urruticoechea: None; C. Perez-Garcia: None; J. Rosas: None; V. Ruiz-Esquide: None; D. Fernández-Lozano: None; I. Brana Abascal: None; E. Cervantes-Perez: None; J. Fernandez-Melon: None; C. Fernandez: None; B. Flores Robles: None; D. Ferrer: None; R. Blanco: AbbVie, 5, 6, Amgen, 6, AstraZeneca, 2, BMS, 6, Eli Lilly, 6, Galapagos, 2, 6, Janssen, 2, 6, MSD, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche, 5, 6, Sanofi, 6.



