Poster Session C
Vasculitis
Session: (2387–2424) Vasculitis – Non-ANCA-Associated & Related Disorders Poster III
2414: Relapse in Giant Cell Arteritis Treated with Tocilizumab. Predictive Factors
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- FL
Fernando López-Gutierrez, PhD
Rheumatology, Hospital Universitario Marqués de Valdecilla
Santander, SpainDisclosure information not submitted.
Abstract Poster Presenter(s)
Fernando López1, Javier Loricera2, Ivan Ferraz Amaro3, Santos Castañeda4, Clara Moriano Morales5, Javier Narvaez6, Vicente Aldasoro7, Olga Maiz8, Rafael Benito Melero-Gonzalez9, Juan I. Villa10, PALOMA VELA11, Susana Romero Yuste12, Jose L. Callejas13, Eugenio De Miguel14, Eva Galindez-Agirregoikoa15, Francisca Sivera16, Jesús Carlos Fernández López17, Arturo Llobell18, Julio Sánchez-Martín19, Calderon Goercke2, Lara Sanchez-Bilbao20, Jose L. Hernández21 and Ricardo Blanco22, 1Rheumatology, Hospital Universitario Marqués de Valdecilla, Santander, Spain, 2Hospital Universitario Marqués de Valdecilla, Santander, Spain, 3Hospital Universitario de Canarias, Santa Cruz de Tenerife, Spain, 4Hospital Universitario de la Princesa, Madrid, Spain, 5Rheumatology, Hospital Universitario de León, León, Spain, 6Hospital Universitario de Bellvitge, Barcelona, Spain, 7Hospital Universitario de Navarra, Pamplona, Spain, 8Hospital Universitario de Donostia. San Sebastián, Spain., Donosti, Spain, 9CHU Vigo, O Carballino, Spain, 10Department of Rheumatology, Hospital Sierrallana, Torrelavega, Torrelavega, Spain, 11Rheumatology, Hospital General Universitario Alicante, Alicante, Spain, 12Complexo Hospitalario Universitario, Pontevedra, Spain, 13Unit of Systemic Autoimmune Diseases, Hospital San Cecilio, Granada, Spain, 14Hospital Universitario La Paz, Madrid, Spain, 15Basurto University Hospital, Bilbao, Spain, 16Elda General University Hospital, Elda, Spain, 17Rheumatology department, Complexo Hospitalario Universitario A Coruña (CHUAC). Instituto de Investigación Biomédica A Coruña (INIBIC), A Coruña, Spain, 18Parc Tauli University Hospital, Barcelona, Spain, 19Department of Rheumatology, Hospital Universitario Marqués de Valdecilla, IDIVAL, Immunopathology Group, Santander, Spain, 20Hospital Universitario Marques de Valdecilla, Santander, Spain, 21Department of Internal Medicine, Hospital Universitario Marqués de Valdecilla, Santander, Spain, 22Hospital Universitario Marqués de Valdecilla, IDIVAL, Santander, Spain
Background/Purpose: Tocilizumab (TCZ) is the only biologic therapy approved for giant cell arteritis (GCA). Clinical trial with TCZ in GCA was performed with intravenous (iv) TCZ in a phase 2 trial (1), and with subcutaneous (sc) TCZ in the phase 3 GiACTA (2). There is general agreement on the initial/maintenance dose, but duration of TCZ therapy is not well established. In GiACTA trial, after one year on TCZ, most patients had GCA relapse after withdrawal.
This study aims to assessthe predictive factors of relapse in GCA in a clinical practice scenario.
Methods: Multicentre observational study of 471 patients with GCA. The diagnosis of GCA was performed between 2016 and 2021 according to: a) ACR criteria, and/or b) temporal artery biopsy, and/or c) imaging techniques. Relapse was defined according to EULAR consensus definition (3). From the 471 patients, we selected the patients who had available the data on relapse during follow-up. Multivariable study was conducted to identify the best set of predictors for the appearance of a relapse.
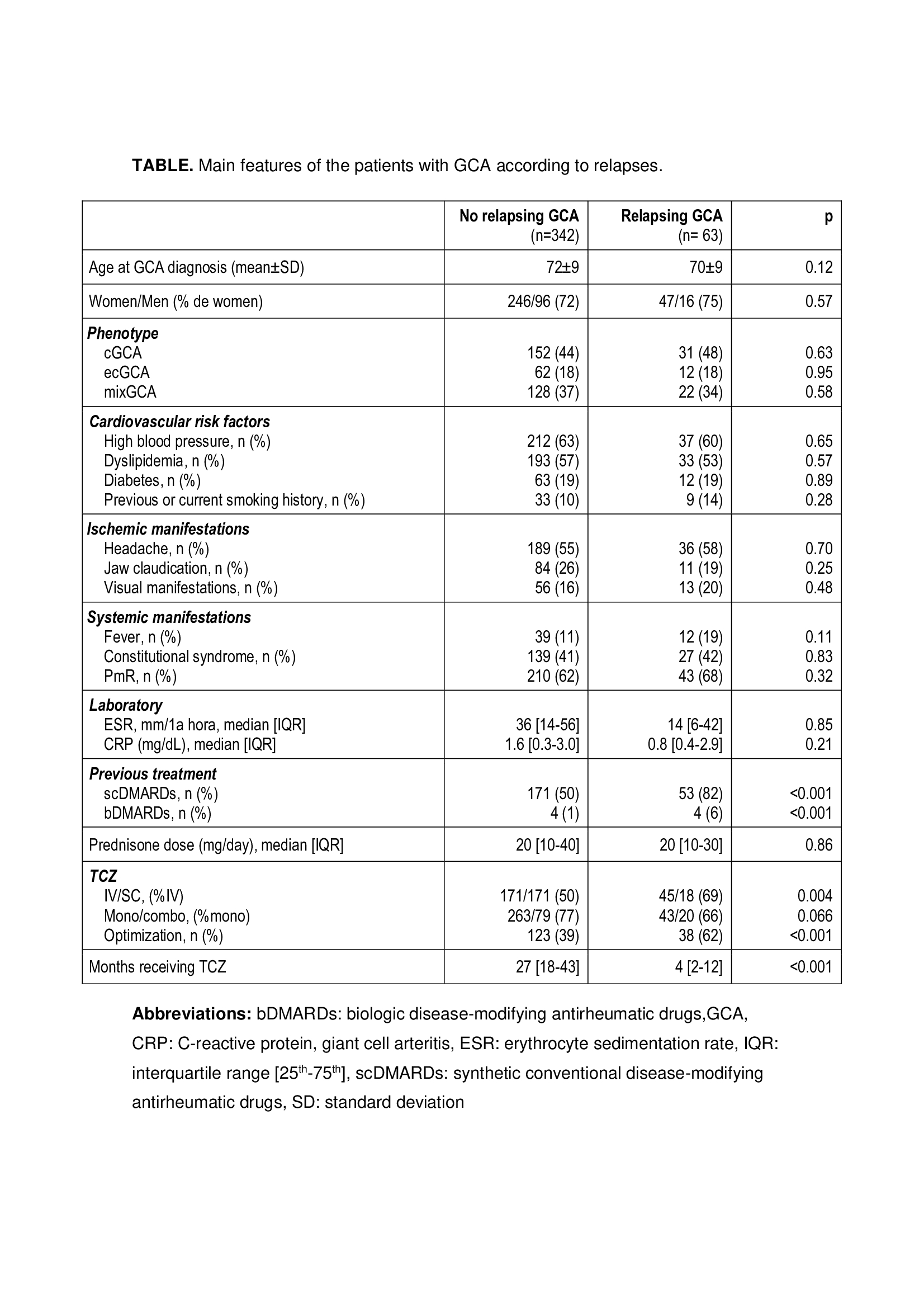
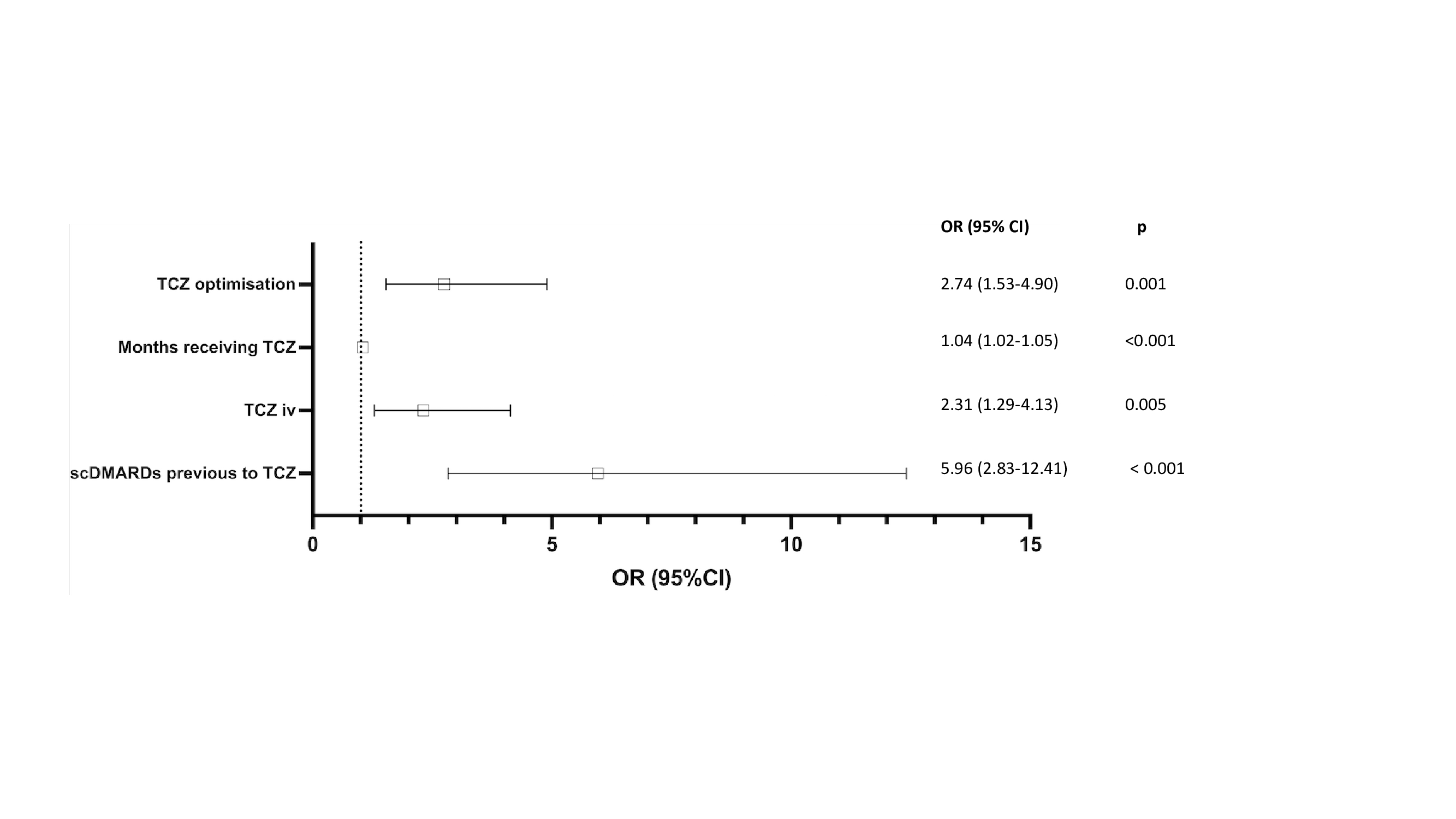
Results: GCA relapses were observed in 63 of 405 (15%) patients for whom such data was available(Table). No significant differences were observed between the two groups in demographic, clinical and laboratory characteristics or in prednisone dose at initiation of TCZ. The set of variables associated with GCA relapses were prior use of synthetic conventional disease-modifying antirheumatic drugs (scDMARDs), use of iv.TCZ, shorter time on TCZ therapy and optimization of TCZ dose (Figure).
Conclusion: GCA relapse seems related mainly to TCZ schedule and was associated with iv TCZ, and a shorter treatment time and optimization. References:
F. López: None; J. Loricera: None; I. Ferraz Amaro: AbbVie/Abbott, 5, 6, Amgen, 5, 6, Bristol-Myers Squibb(BMS), 6; S. Castañeda: None; C. Moriano Morales: None; J. Narvaez: None; V. Aldasoro: None; O. Maiz: None; R. Melero-Gonzalez: None; J. Villa: None; P. VELA: AbbVie/Abbott, 5, AstraZeneca, 5, Eli Lilly, 5, 6, GlaxoSmithKlein(GSK), 6, Novartis, 5, Pfizer, 5; S. Romero Yuste: AbbVie, 6, AstraZeneca, 6, Biogen, 6, Lilly, 5, 6, Pfizer, 6, Sanofi, 1; J. Callejas: None; E. De Miguel: None; E. Galindez-Agirregoikoa: None; F. Sivera: AbbVie/Abbott, 1, AstraZeneca, 5, Bristol-Myers Squibb(BMS), 5, Eli Lilly, 5, GlaxoSmithKlein(GSK), 6, Novartis, 5, 6, Pfizer, 1, Roche, 5, UCB, 6; J. Fernández López: None; A. Llobell: None; J. Sánchez-Martín: None; C. Goercke: None; L. Sanchez-Bilbao: None; J. Hernández: None; R. Blanco: AbbVie, 5, 6, Amgen, 6, AstraZeneca, 2, BMS, 6, Eli Lilly, 6, Galapagos, 2, 6, Janssen, 2, 6, MSD, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche, 5, 6, Sanofi, 6.
Background/Purpose: Tocilizumab (TCZ) is the only biologic therapy approved for giant cell arteritis (GCA). Clinical trial with TCZ in GCA was performed with intravenous (iv) TCZ in a phase 2 trial (1), and with subcutaneous (sc) TCZ in the phase 3 GiACTA (2). There is general agreement on the initial/maintenance dose, but duration of TCZ therapy is not well established. In GiACTA trial, after one year on TCZ, most patients had GCA relapse after withdrawal.
This study aims to assessthe predictive factors of relapse in GCA in a clinical practice scenario.
Methods: Multicentre observational study of 471 patients with GCA. The diagnosis of GCA was performed between 2016 and 2021 according to: a) ACR criteria, and/or b) temporal artery biopsy, and/or c) imaging techniques. Relapse was defined according to EULAR consensus definition (3). From the 471 patients, we selected the patients who had available the data on relapse during follow-up. Multivariable study was conducted to identify the best set of predictors for the appearance of a relapse.
Results: GCA relapses were observed in 63 of 405 (15%) patients for whom such data was available(Table). No significant differences were observed between the two groups in demographic, clinical and laboratory characteristics or in prednisone dose at initiation of TCZ. The set of variables associated with GCA relapses were prior use of synthetic conventional disease-modifying antirheumatic drugs (scDMARDs), use of iv.TCZ, shorter time on TCZ therapy and optimization of TCZ dose (Figure).
Conclusion: GCA relapse seems related mainly to TCZ schedule and was associated with iv TCZ, and a shorter treatment time and optimization. References:
- Villiger PM, et al. Lancet. 2016. PMID: 26952547
- Stone JH, et al. N Engl J Med. 2017. PMID: 28745999
- Hellmich B, et al. Ann Rheum Dis. 2020. PMID: 31270110

Main features of the patients with GCA according to relapses.

F. López: None; J. Loricera: None; I. Ferraz Amaro: AbbVie/Abbott, 5, 6, Amgen, 5, 6, Bristol-Myers Squibb(BMS), 6; S. Castañeda: None; C. Moriano Morales: None; J. Narvaez: None; V. Aldasoro: None; O. Maiz: None; R. Melero-Gonzalez: None; J. Villa: None; P. VELA: AbbVie/Abbott, 5, AstraZeneca, 5, Eli Lilly, 5, 6, GlaxoSmithKlein(GSK), 6, Novartis, 5, Pfizer, 5; S. Romero Yuste: AbbVie, 6, AstraZeneca, 6, Biogen, 6, Lilly, 5, 6, Pfizer, 6, Sanofi, 1; J. Callejas: None; E. De Miguel: None; E. Galindez-Agirregoikoa: None; F. Sivera: AbbVie/Abbott, 1, AstraZeneca, 5, Bristol-Myers Squibb(BMS), 5, Eli Lilly, 5, GlaxoSmithKlein(GSK), 6, Novartis, 5, 6, Pfizer, 1, Roche, 5, UCB, 6; J. Fernández López: None; A. Llobell: None; J. Sánchez-Martín: None; C. Goercke: None; L. Sanchez-Bilbao: None; J. Hernández: None; R. Blanco: AbbVie, 5, 6, Amgen, 6, AstraZeneca, 2, BMS, 6, Eli Lilly, 6, Galapagos, 2, 6, Janssen, 2, 6, MSD, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche, 5, 6, Sanofi, 6.



