Poster Session C
Vasculitis
Session: (2387–2424) Vasculitis – Non-ANCA-Associated & Related Disorders Poster III
2413: Janus Kinase Inhibitors in Giant Cell Arteritis in Clinical Practice. Real-World Clinical Practice Study and Literature Review
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- FL
Fernando López-Gutierrez, PhD
Rheumatology, Hospital Universitario Marqués de Valdecilla
Santander, SpainDisclosure information not submitted.
Abstract Poster Presenter(s)
Fernando López1, Javier Loricera2, Toluwalase Tofade3, Diana Prieto-Peña2, Susana Romero Yuste4, Eugenio De Miguel5, Anne Riveros-Frutos6, Ivan Ferraz Amaro7, Santos Castañeda8, Eztizen Labrador-Sánchez9, Olga Maiz10, Elena Becerra-Fernández11, Javier Narvaez12, Eva Galindez-Agirregoikoa13, Ismael González14, Ana Urruticoechea15, Sebastian Unizony16 and Ricardo Blanco17, 1Rheumatology, Hospital Universitario Marqués de Valdecilla, Santander, Spain, 2Hospital Universitario Marqués de Valdecilla, Santander, Spain, 3Neurology Department, Massachusetts General Hospital, Boston, MA, 4Complexo Hospitalario Universitario, Pontevedra, Spain, 5Hospital Universitario La Paz, Madrid, Spain, 6Hospital Universitario Germans Trias i Pujol, Barcelona, Spain, 7Hospital Universitario de Canarias, Santa Cruz de Tenerife, Spain, 8Hospital Universitario de la Princesa, Madrid, Spain, 9Hospital Universitario San Pedro, Laguardia, Spain, 10Hospital Universitario de Donostia. San Sebastián, Spain., Donosti, Spain, 11Department of Rheumatology, Hospital Universitario de Torrevieja, London, United Kingdom, 12Hospital Universitario de Bellvitge, Barcelona, Spain, 13Basurto University Hospital, Bilbao, Spain, 14Hospital Universitario de León, León, Spain, 15Hospital Can Misses, Ibiza, Spain, 16Massachusetts General Hospital, Winchester, MA, 17Hospital Universitario Marqués de Valdecilla, IDIVAL, Santander, Spain
Background/Purpose: Patients with giant cell arteritis (GCA) can relapse despite glucocorticoids, methotrexate and tocilizumab treatment. The JAK/STAT signalling pathway is involved in the pathogenesis of GCA, and JAK inhibitors (JAKi) are a potential treatment alternative. Baricitinib showed positive results in a small uncontrolled study (1).
The objective of this study is to evaluate the effectiveness of JAKi in GCA.
Methods: Real-world, retrospective clinical practice study of patients with GCA treated with JAKi. Outcomes assessed included disease relapse and safety. A literature search for other JAKi-treated GCA cases was conducted in PubMed, Embase and the Cochrane library from inception to 04/30/2023. We compared results of the previous baricitinib study (1) and the baricitinib recipients in our series.
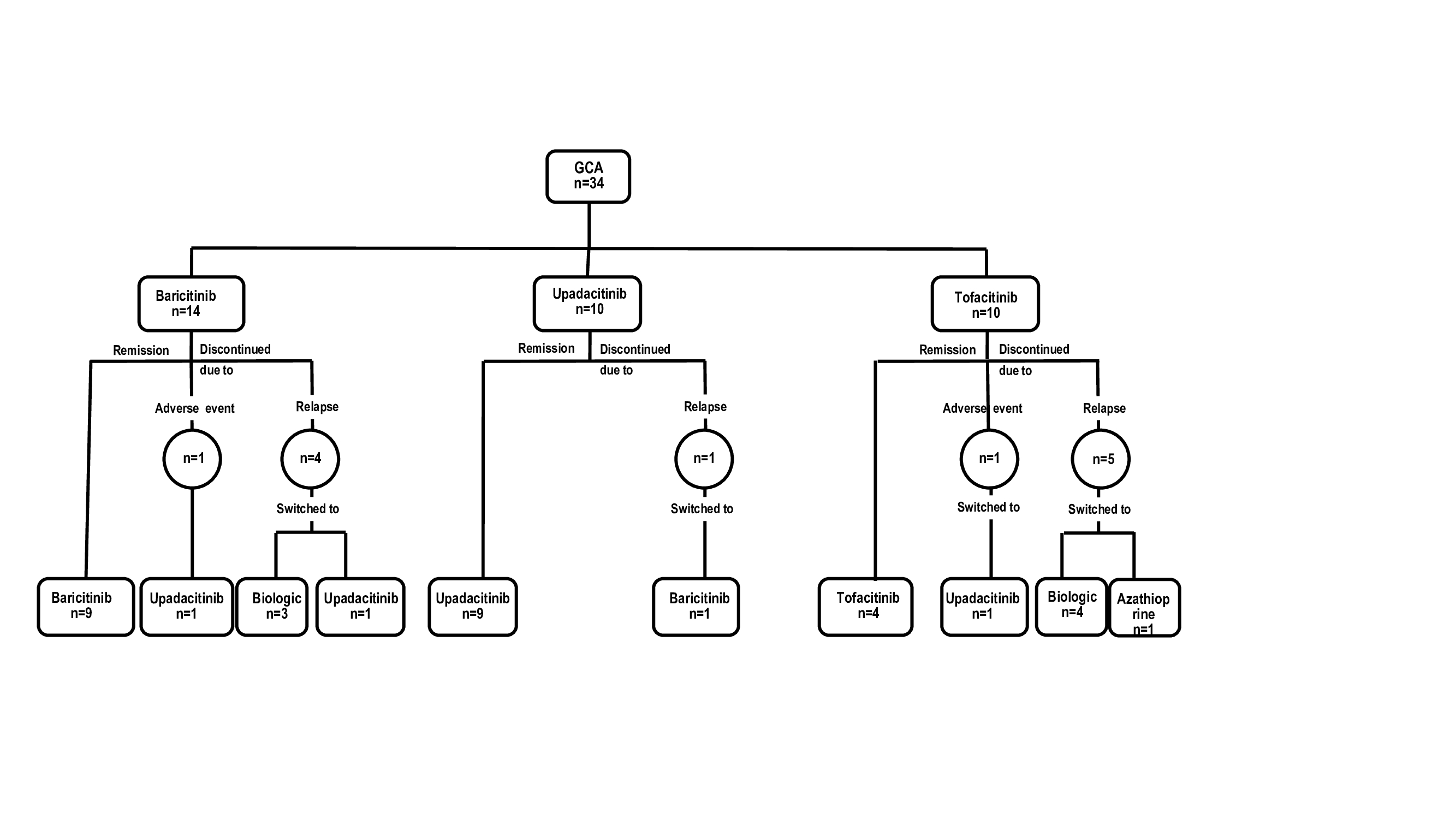
Results: We present 34 patients (29 females[85%], mean age, 72.2 years, relapsing disease 34 [100%]) that received JAKi . The initial JAKi was baricitinib (n=14), tofacitinib (n=10) and upadacitinib (n=10) (Table and Figure). After a median [IQR] follow-up of 9.5 [4.2-12.7] months, 22 (64.7%) achieved and maintained remission, and 12 (35.3%) patients discontinued the initial JAKi due to relapse (n=10, 29.4%) or severe adverse events (SAEs) (n=2, 5.9%) including liver dysfunction and dyspnea/palpitations. The 12 patients failing the initial JAKi were switched to an alternative [JAKi (n=4), biologic therapy (n=7) and azathioprine (n=1)]. The literature review identified another 21 GCA patients (17 females, mean age 74.2 years) treated with JAKi, mostly with baricitinib (n=18). Most of these patients benefited from JAKi therapy (Table). Patients in our series receiving baricitinib had longer disease duration (median [IQR]31 [12-51] vs 9 [7-21] months; p=0.001) and had received biologics (71% vs 6.7%; p< 0.001) more frequently than those in the previous baricitinib study (1). Remaining baseline features were similar.
Conclusion: This real-world analysis suggest that JAKi could be effective in GCA, including patients failing other immunosuppressive therapies. The results of an ongoing phase 3 randomized controlled trial are awaited to confirm or rule out this observation.
References:
.jpg)
F. López: None; J. Loricera: None; T. Tofade: None; D. Prieto-Peña: None; S. Romero Yuste: AbbVie, 6, AstraZeneca, 6, Biogen, 6, Lilly, 5, 6, Pfizer, 6, Sanofi, 1; E. De Miguel: None; A. Riveros-Frutos: None; I. Ferraz Amaro: AbbVie/Abbott, 5, 6, Amgen, 5, 6, Bristol-Myers Squibb(BMS), 6; S. Castañeda: None; E. Labrador-Sánchez: None; O. Maiz: None; E. Becerra-Fernández: None; J. Narvaez: None; E. Galindez-Agirregoikoa: None; I. González: None; A. Urruticoechea: None; S. Unizony: None; R. Blanco: AbbVie, 5, 6, Amgen, 6, AstraZeneca, 2, BMS, 6, Eli Lilly, 6, Galapagos, 2, 6, Janssen, 2, 6, MSD, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche, 5, 6, Sanofi, 6.
Background/Purpose: Patients with giant cell arteritis (GCA) can relapse despite glucocorticoids, methotrexate and tocilizumab treatment. The JAK/STAT signalling pathway is involved in the pathogenesis of GCA, and JAK inhibitors (JAKi) are a potential treatment alternative. Baricitinib showed positive results in a small uncontrolled study (1).
The objective of this study is to evaluate the effectiveness of JAKi in GCA.
Methods: Real-world, retrospective clinical practice study of patients with GCA treated with JAKi. Outcomes assessed included disease relapse and safety. A literature search for other JAKi-treated GCA cases was conducted in PubMed, Embase and the Cochrane library from inception to 04/30/2023. We compared results of the previous baricitinib study (1) and the baricitinib recipients in our series.
Results: We present 34 patients (29 females[85%], mean age, 72.2 years, relapsing disease 34 [100%]) that received JAKi . The initial JAKi was baricitinib (n=14), tofacitinib (n=10) and upadacitinib (n=10) (Table and Figure). After a median [IQR] follow-up of 9.5 [4.2-12.7] months, 22 (64.7%) achieved and maintained remission, and 12 (35.3%) patients discontinued the initial JAKi due to relapse (n=10, 29.4%) or severe adverse events (SAEs) (n=2, 5.9%) including liver dysfunction and dyspnea/palpitations. The 12 patients failing the initial JAKi were switched to an alternative [JAKi (n=4), biologic therapy (n=7) and azathioprine (n=1)]. The literature review identified another 21 GCA patients (17 females, mean age 74.2 years) treated with JAKi, mostly with baricitinib (n=18). Most of these patients benefited from JAKi therapy (Table). Patients in our series receiving baricitinib had longer disease duration (median [IQR]31 [12-51] vs 9 [7-21] months; p=0.001) and had received biologics (71% vs 6.7%; p< 0.001) more frequently than those in the previous baricitinib study (1). Remaining baseline features were similar.
Conclusion: This real-world analysis suggest that JAKi could be effective in GCA, including patients failing other immunosuppressive therapies. The results of an ongoing phase 3 randomized controlled trial are awaited to confirm or rule out this observation.
References:
- Koster MJ, et al. Ann Rheum Dis. 2022
.jpg)
Current series and literature review of patients with GCA treated with JAKi.

Flow chart of the 34 GCA patients treated with JAKi
F. López: None; J. Loricera: None; T. Tofade: None; D. Prieto-Peña: None; S. Romero Yuste: AbbVie, 6, AstraZeneca, 6, Biogen, 6, Lilly, 5, 6, Pfizer, 6, Sanofi, 1; E. De Miguel: None; A. Riveros-Frutos: None; I. Ferraz Amaro: AbbVie/Abbott, 5, 6, Amgen, 5, 6, Bristol-Myers Squibb(BMS), 6; S. Castañeda: None; E. Labrador-Sánchez: None; O. Maiz: None; E. Becerra-Fernández: None; J. Narvaez: None; E. Galindez-Agirregoikoa: None; I. González: None; A. Urruticoechea: None; S. Unizony: None; R. Blanco: AbbVie, 5, 6, Amgen, 6, AstraZeneca, 2, BMS, 6, Eli Lilly, 6, Galapagos, 2, 6, Janssen, 2, 6, MSD, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche, 5, 6, Sanofi, 6.



