Poster Session C
Rheumatoid arthritis (RA)
Session: (2141–2176) RA – Treatment Poster III
2164: Real-world Persistence of Initial Targeted Therapy Strategy in Monotherapy versus Combination Therapy in Patients with Rheumatoid Arthritis, Psoriatic Arthritis, and Ankylosing Spondylitis
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- ZP
Zulema Plaza, PhD, MSc
Fundacion Española de Reumatología
Madrid, SpainDisclosure information not submitted.
Abstract Poster Presenter(s)
Carlos Sánchez-Piedra1, Lorena Expósito2, PALOMA VELA3, Manuel José Moreno Ramos4, Cristina Campos5, Cristina Bohorquez6, Jerusalem Calvo7, Zulema Plaza8, Marta Domínguez9 and Jose Federico Diaz-Gonzalez10, 1Health Technology Assessment Agency (AETS), Instituto de Salud Carlos III, Madrid, Spain, 2Rheumatology Unit, Hospital Universitario de Canarias, San Cristóbal de La Laguna, Spain, 3Rheumatology, Hospital General Universitario Alicante, Alicante, Spain, 4Rheumatology Department Hospital Virgen de la Arrixaca, El Palmar Murcia, Spain, 5Rheumatology Unit, Hospital General Universitario de Valencia, Valencia, Spain, 6Rheumatology Unit, Hospital Universitario Príncipe de Asturias, Alcalá de Henares, Spain, 7Reina Sofia University Hospital, Córdoba, Spain, 8Universidad Autónoma de Madrid, Madrid, Spain, 9Sociedad Española de Reumatología, Madrid, Spain, 10Hospital Universitario de Canarias, La Laguna, Spain
Background/Purpose: Clinical practice guidelines, based on information from clinical trials, provide different recommendations for the use of combination therapies for the treatment of rheumatoid arthritis (RA), ankylosing spondylitis (AS), and psoriatic arthritis (PsA). Few studies have evaluated the persistence of different treatment strategies (combination vs. monotherapy) as a primary endpoint based on data from real-world settings. The aim of this study was to evaluate the persistence of the initial strategy with or without conventional synthetic (cs) disease-modifying antirheumatic drugs (DMARDs) (combination and monotherapy strategies, respectively) of targeted therapy in patients with RA, AS and PsA under real-life conditions. Factors associated with maintenance of the initial strategy were also analyzed.
Methods: Nested cohort study within BIOBADASER III, a prospective Spanish registry of patients with rheumatic diseases treated with targeted therapy, including biologic(b) and targeted synthetic(ts) DMARDs. Bivariate comparisons and multivariate Cox proportional hazards models were used for the analyses.
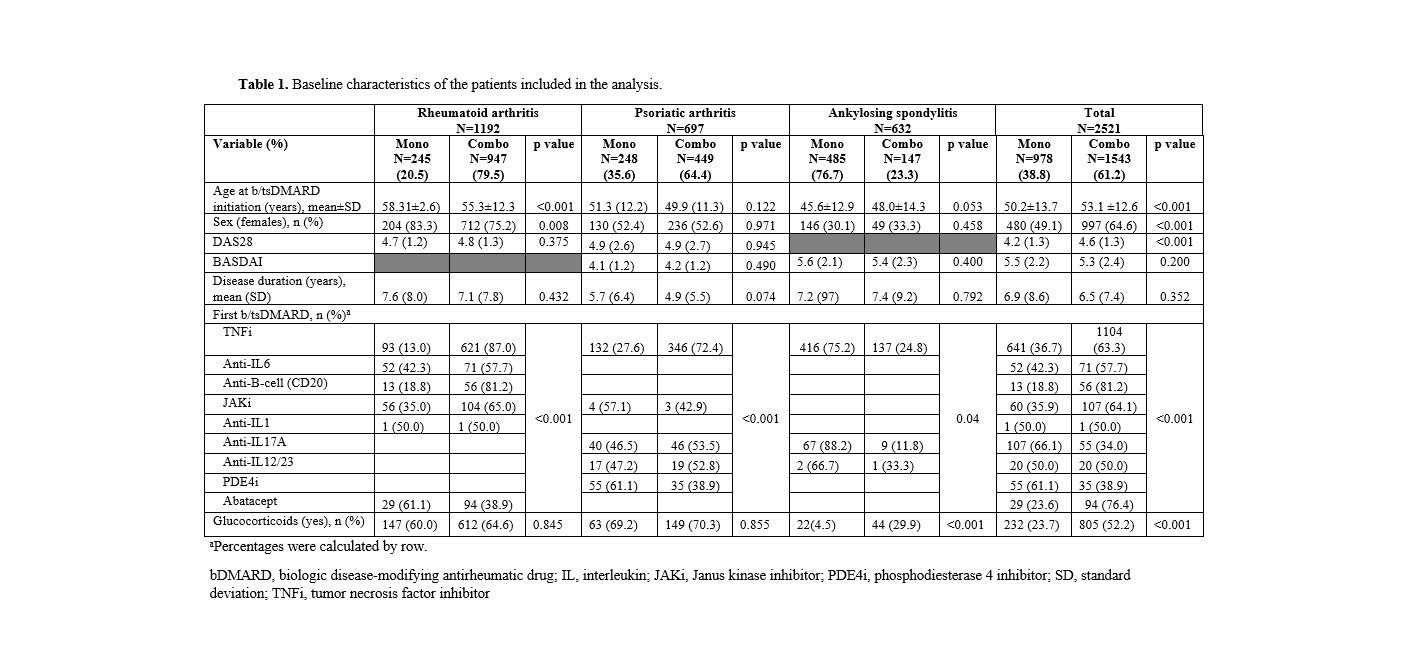
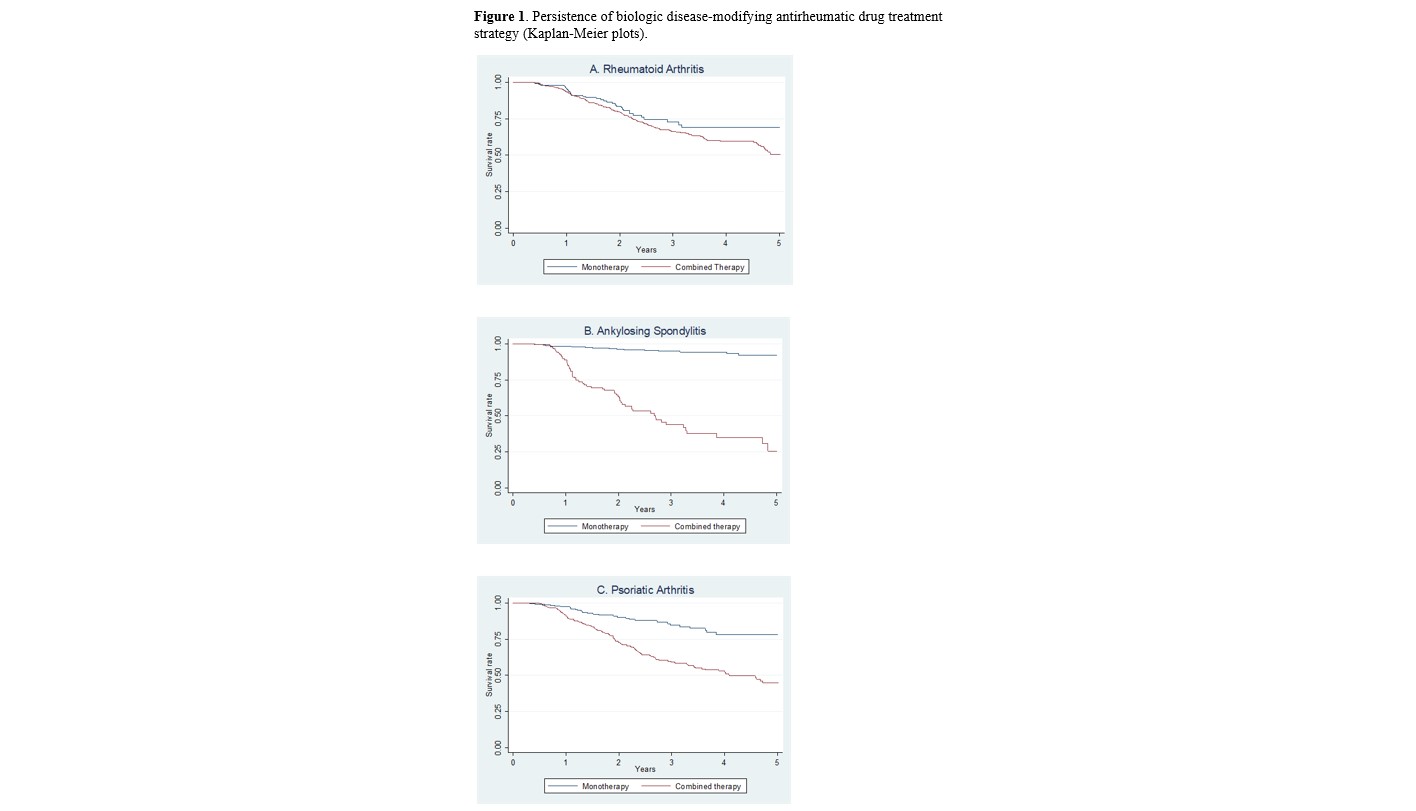
Results: A total of 2,521 patients were included in the study. Baseline demographics and disease characteristics, disease activity, type of targeted therapy, and concomitant medications are shown in Table 1. In the multivariate model, the initial strategy of combination therapy was associated with shorter persistence in patients with RA (hazard ratio [HR] 1.58;95% confidence interval [CI] 1.00 to 2.50; p=0.049), PsA (HR 2.48; 95%CI 1.65 to 3.72) and AS (HR 16.77; 95%CI 7.37 to 38.16; p< 0.001). 48; 95%CI 1.65 to 3.72; p< 0.001) and AS (HR 16.77; 95%CI 7.37 to 38.16; p< 0.001), regardless of age, sex, time of disease progression, baseline disease activity or type of b/tsDMARD. Figure 1 shows the analyses of persistence by disease using Kaplan-Meier curves. Overall, the combination strategy was associated with an increased incidence of adverse events (incidence rate ratio [IRR] 1.13; 95%CI 1.05-1.21).
Conclusion: Analysis of a large database collected under real-world conditions shows that initiation of targeted therapy in monotherapy has a significantly better persistence and safety profile than in combination with csDMARD(s) in patients with PsA and AS. In patients with RA, the results also suggest that monotherapy should be considered as a therapeutic option as it offers a higher chance of persistence.
C. Sánchez-Piedra: None; L. Expósito: None; P. VELA: AbbVie/Abbott, 5, AstraZeneca, 5, Eli Lilly, 5, 6, GlaxoSmithKlein(GSK), 6, Novartis, 5, Pfizer, 5; M. Moreno Ramos: None; C. Campos: None; C. Bohorquez: None; J. Calvo: None; Z. Plaza: None; M. Domínguez: None; J. Diaz-Gonzalez: None.
Background/Purpose: Clinical practice guidelines, based on information from clinical trials, provide different recommendations for the use of combination therapies for the treatment of rheumatoid arthritis (RA), ankylosing spondylitis (AS), and psoriatic arthritis (PsA). Few studies have evaluated the persistence of different treatment strategies (combination vs. monotherapy) as a primary endpoint based on data from real-world settings. The aim of this study was to evaluate the persistence of the initial strategy with or without conventional synthetic (cs) disease-modifying antirheumatic drugs (DMARDs) (combination and monotherapy strategies, respectively) of targeted therapy in patients with RA, AS and PsA under real-life conditions. Factors associated with maintenance of the initial strategy were also analyzed.
Methods: Nested cohort study within BIOBADASER III, a prospective Spanish registry of patients with rheumatic diseases treated with targeted therapy, including biologic(b) and targeted synthetic(ts) DMARDs. Bivariate comparisons and multivariate Cox proportional hazards models were used for the analyses.
Results: A total of 2,521 patients were included in the study. Baseline demographics and disease characteristics, disease activity, type of targeted therapy, and concomitant medications are shown in Table 1. In the multivariate model, the initial strategy of combination therapy was associated with shorter persistence in patients with RA (hazard ratio [HR] 1.58;95% confidence interval [CI] 1.00 to 2.50; p=0.049), PsA (HR 2.48; 95%CI 1.65 to 3.72) and AS (HR 16.77; 95%CI 7.37 to 38.16; p< 0.001). 48; 95%CI 1.65 to 3.72; p< 0.001) and AS (HR 16.77; 95%CI 7.37 to 38.16; p< 0.001), regardless of age, sex, time of disease progression, baseline disease activity or type of b/tsDMARD. Figure 1 shows the analyses of persistence by disease using Kaplan-Meier curves. Overall, the combination strategy was associated with an increased incidence of adverse events (incidence rate ratio [IRR] 1.13; 95%CI 1.05-1.21).
Conclusion: Analysis of a large database collected under real-world conditions shows that initiation of targeted therapy in monotherapy has a significantly better persistence and safety profile than in combination with csDMARD(s) in patients with PsA and AS. In patients with RA, the results also suggest that monotherapy should be considered as a therapeutic option as it offers a higher chance of persistence.

Table 1. Baseline characteristics of the patients included in the analysis.

Figure 1. Persistence of biologic disease-modifying antirheumatic drug treatment strategy (Kaplan-Meier plots).
C. Sánchez-Piedra: None; L. Expósito: None; P. VELA: AbbVie/Abbott, 5, AstraZeneca, 5, Eli Lilly, 5, 6, GlaxoSmithKlein(GSK), 6, Novartis, 5, Pfizer, 5; M. Moreno Ramos: None; C. Campos: None; C. Bohorquez: None; J. Calvo: None; Z. Plaza: None; M. Domínguez: None; J. Diaz-Gonzalez: None.



