Poster Session C
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (2227–2256) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III: SpA
2251: Direct and Indirect Effects of Upadacitinib or Adalimumab on Pain in Psoriatic Arthritis: Results from a Randomized Phase 3 Study
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- PT
Peter Taylor, PhD, FRCP, MA
University of Oxford
Oxford, United KingdomDisclosure information not submitted.
Abstract Poster Presenter(s)
Peter C. Taylor1, David Walsh2, Tsutomu Takeuchi3, Bruno Fautrel4, Janet Pope5, Koji Kato6, Arathi Setty6, Tianming Gao6, Diane Caballero7, Ralph Lippe8 and Arthur Kavanaugh9, 1Nuffield Department of Orthopedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, United Kingdom, 2University of Nottingham, Nottingham, United Kingdom, 3Keio University School of Medicine and Saitama Medical University, Tokyo, Japan, 4Sorbonne Université APHP, Paris, France, 5University of Western Ontario, London, ON, Canada, 6AbbVie, Inc., North Chicago, IL, 7AbbVie, Inc., Chicago, IL, 8AbbVie Deutschland GmbH & Co. KG, Wiesbaden, Germany, 9University of California San Diego, School of Medicine, Riverside, CA
Background/Purpose: Pain, including inflammatory joint pain, may be debilitating in patients with psoriatic arthritis (PsA). Pain in PsA is multidimensional and can be influenced via reduction of inflammation and potentially by other means, such as a direct analgesic drug effect. The objective of this analysis of the SELECT-PsA 1 study was to assess the direct and indirect (ie, by changes in inflammation surrogates) effects of treatment with upadacitinib (UPA), a selective and reversible Janus kinase (JAK) inhibitor, or adalimumab (ADA), a TNF inhibitor, vs placebo (PBO) on pain in patients with PsA.
Methods: SELECT-PsA 1 was a randomized, double-blind phase 3 study in patients with PsA who had active disease at baseline. Adults (≥18 years) were randomized 1:1:1:1 to UPA 15 mg once daily, UPA 30 mg once daily, ADA 40 mg every other week, or PBO; for UPA, only UPA 15 mg data are reported here. As observed analysis was used for change from baseline to week 16 in Patient's Global Assessment (PtGA) of pain (1–100 mm) or tender joint count based on 28 joints (TJC28). Observed case multiple mediation analysis1 for effect of UPA vs PBO and ADA vs PBO on pain (pain assessed as PtGA of pain or TJC28) was conducted. Indirect effect of treatment on pain was assessed based on inflammatory factors including itch, total enthesitis, Leeds Enthesitis Index (LEI), and CRP.
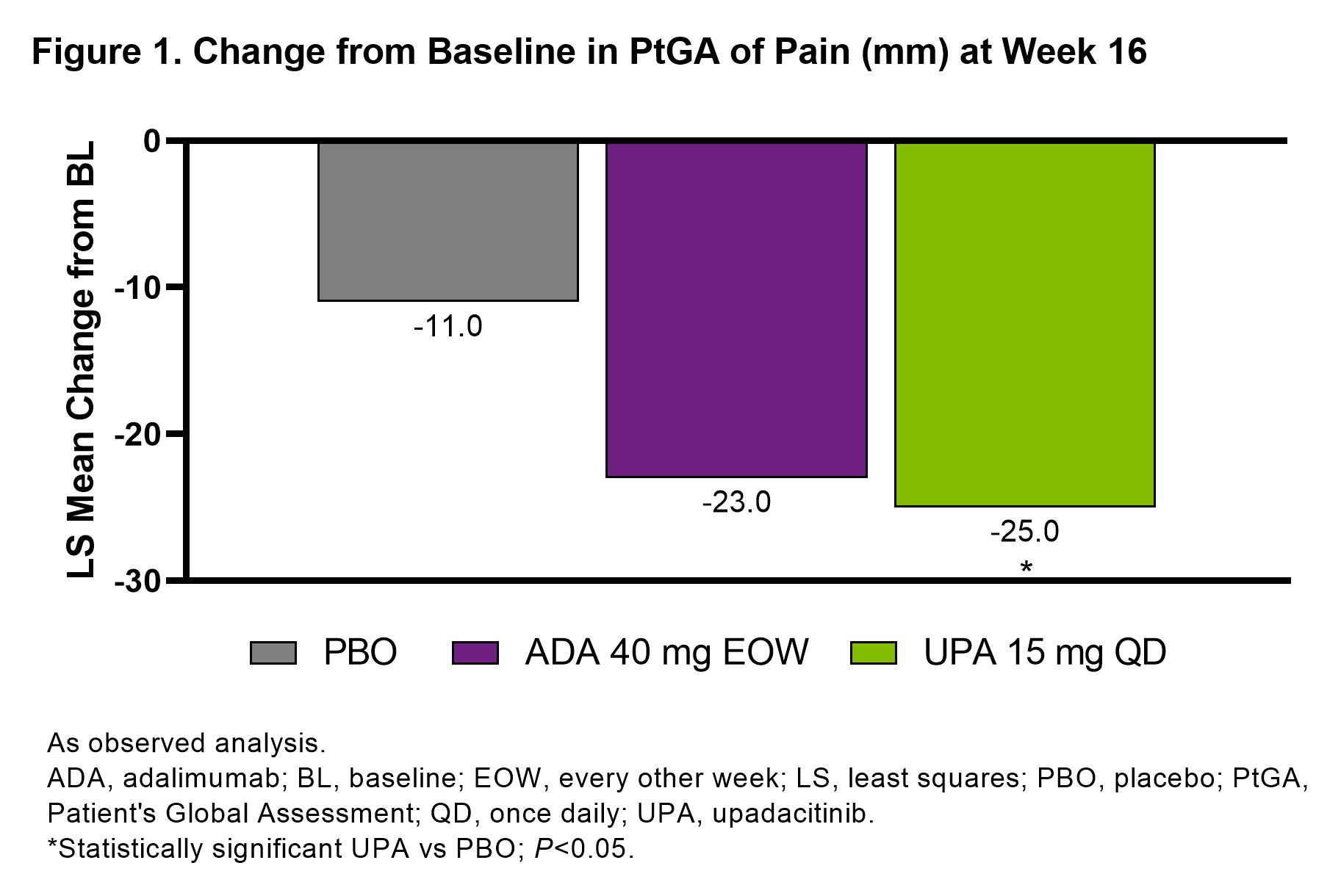
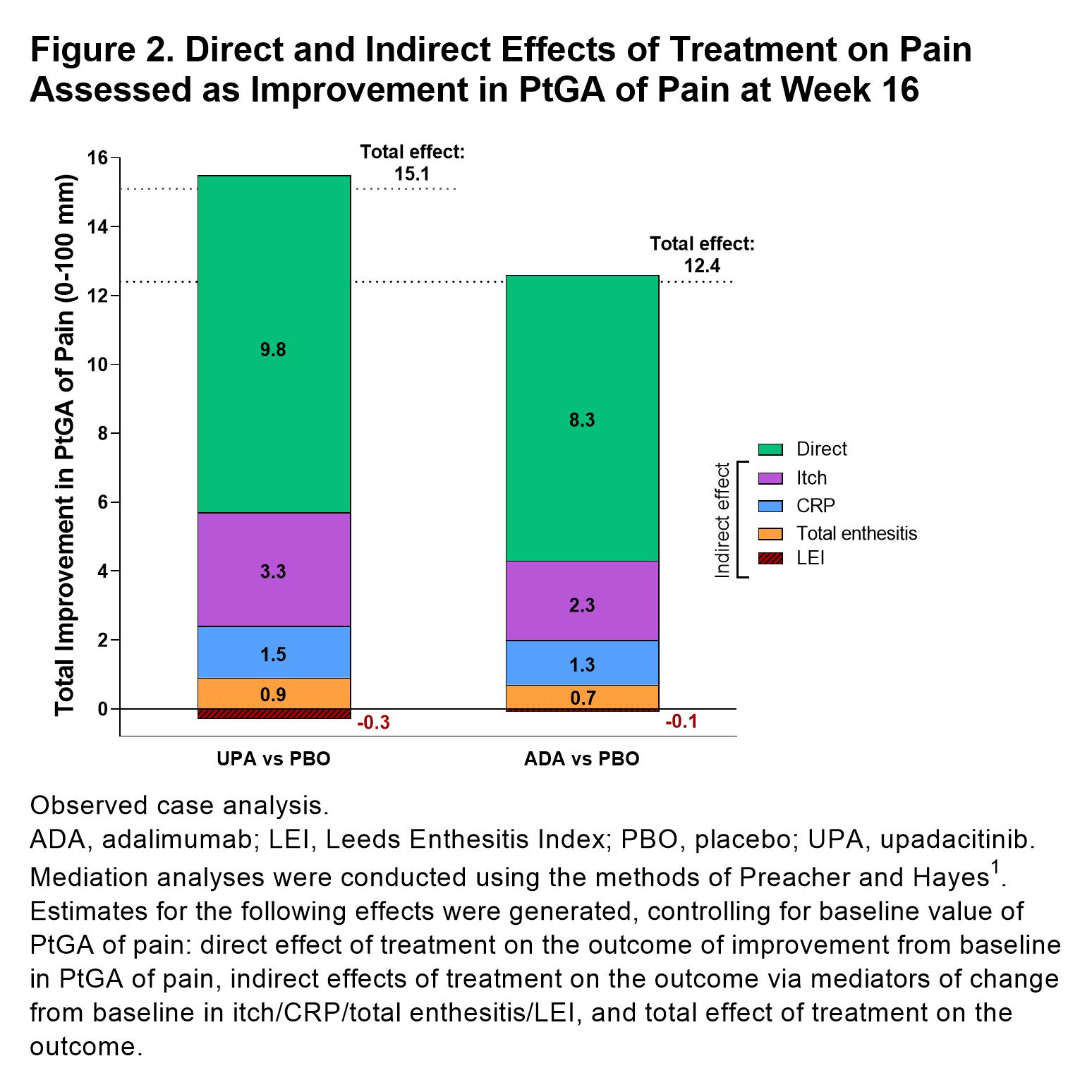
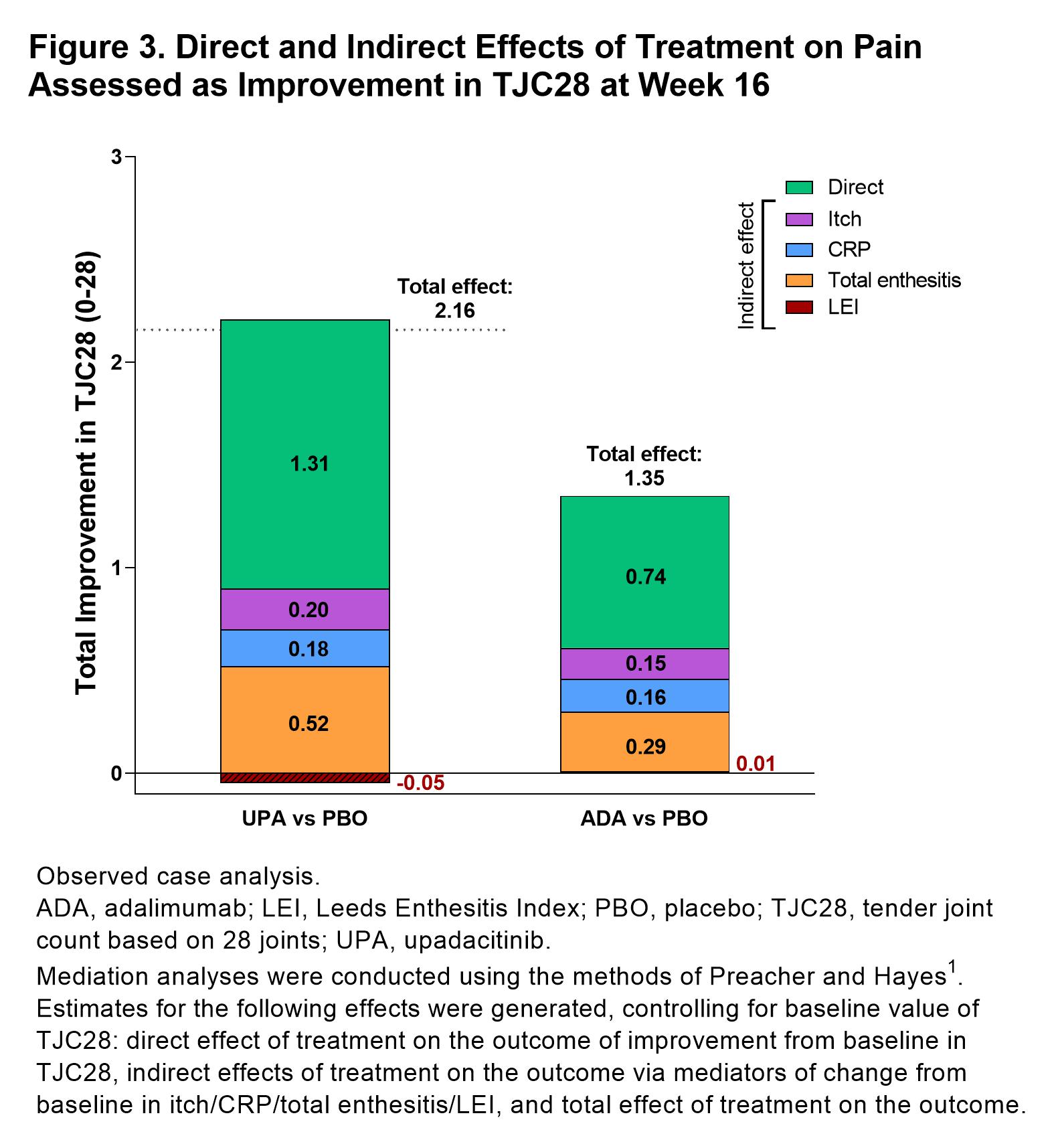
Results: 1281 patients were included in this analysis (UPA, n=429, ADA, n=429, PBO, n=423).PtGA of pain significantly improved with UPA vs PBO from baseline to week 16 (–25.0 [n=404] vs –11.0 [n=390]; P< 0.05; Figure 1). Improvements in pain were also observed with ADA at week 16 (–23.0 [n=408]). Total effects (15.1 and 12.4) and direct effects (9.8 and 8.3) on improvement in PtGA of pain were significantly greater with both UPA vs PBO and ADA vs PBO, respectively at week 16 (all P< 0.001; Figure 2); numerically greater reductions in total effect on pain were observed with UPA vs ADA. Direct and indirect effects on pain assessed as improvement in PtGA of pain were numerically greater with UPA vs ADA (Figure 2). Improvement in elicited pain assessed asTJC28 was also significantly greater with UPA and ADA vs PBO (all P< 0.05; Figure 3). Furthermore, UPA reduced pain assessed as TJC28 numerically more than ADA when examining the total effect explained by direct effects and the inflammatory mediators (indirect effects).
Conclusion: UPA and ADA produced relevantly higher mean improvements in pain via inflammatory or non-inflammatory mechanisms vs PBO in patients with PsA. Total effects on pain improvement were numerically greater with UPA vs ADA when assessed as PtGA of pain and TJC28.
Reference: 1Preacher, KJ & Hayes, AF. Behavior Research Methods,2008;40(3), 879–891.
P. Taylor: AbbVie, 2, Biogen, 2, Eli Lilly, 2, Fresenius, 2, Galapagos, 2, 5, Gilead Sciences, 2, GSK, 2, Janssen, 2, Nordic Pharma, 2, Pfizer Inc, 2, Sanofi, 2, UCB, 2; D. Walsh: AbbVie, 2, Contura International A/S, 2, Eli Lilly, 5, GlaxoSmithKline Research & Development Limited, 5, Orion Corporation, 5, Pfizer, 5; T. Takeuchi: AbbVie, 2, 5, 6, AYUMI, 5, Bristol-Myers Squibb, 6, Chugai, 2, 5, 6, Daiichi Sankyo, 5, Eisai, 5, 6, Eli Lilly Japan, 2, 6, Gilead, 2, 6, Janssen, 6, Mitsubishi-Tanabe, 2, 5, 6, ONO, 5, Pfizer Japan, 6, Taiho, 2; B. Fautrel: AbbVie, 2, BMS, 2, Chugai, 2, Fresenius Kabi, 2, Galapagos, 2, Lilly, 2, Medac, 2, Nordic Pharma, 2, Novartis, 2, Pfizer, 2, Sobi, 2, UCB, 2; J. Pope: AbbVie, 1, 2; K. Kato: AbbVie/Abbott, 3, 11; A. Setty: AbbVie, 3, 11; T. Gao: AbbVie, 3, 11; D. Caballero: AbbVie, 3, 11; R. Lippe: AbbVie, 3, 11; A. Kavanaugh: AbbVie, 1, 2, Amgen, 1, 2, BMS, 1, 2, Eli Lilly, 1, 2, Novartis, 1, 2, Pfizer, 1, 2, UCB, 1, 2.
Background/Purpose: Pain, including inflammatory joint pain, may be debilitating in patients with psoriatic arthritis (PsA). Pain in PsA is multidimensional and can be influenced via reduction of inflammation and potentially by other means, such as a direct analgesic drug effect. The objective of this analysis of the SELECT-PsA 1 study was to assess the direct and indirect (ie, by changes in inflammation surrogates) effects of treatment with upadacitinib (UPA), a selective and reversible Janus kinase (JAK) inhibitor, or adalimumab (ADA), a TNF inhibitor, vs placebo (PBO) on pain in patients with PsA.
Methods: SELECT-PsA 1 was a randomized, double-blind phase 3 study in patients with PsA who had active disease at baseline. Adults (≥18 years) were randomized 1:1:1:1 to UPA 15 mg once daily, UPA 30 mg once daily, ADA 40 mg every other week, or PBO; for UPA, only UPA 15 mg data are reported here. As observed analysis was used for change from baseline to week 16 in Patient's Global Assessment (PtGA) of pain (1–100 mm) or tender joint count based on 28 joints (TJC28). Observed case multiple mediation analysis1 for effect of UPA vs PBO and ADA vs PBO on pain (pain assessed as PtGA of pain or TJC28) was conducted. Indirect effect of treatment on pain was assessed based on inflammatory factors including itch, total enthesitis, Leeds Enthesitis Index (LEI), and CRP.
Results: 1281 patients were included in this analysis (UPA, n=429, ADA, n=429, PBO, n=423).PtGA of pain significantly improved with UPA vs PBO from baseline to week 16 (–25.0 [n=404] vs –11.0 [n=390]; P< 0.05; Figure 1). Improvements in pain were also observed with ADA at week 16 (–23.0 [n=408]). Total effects (15.1 and 12.4) and direct effects (9.8 and 8.3) on improvement in PtGA of pain were significantly greater with both UPA vs PBO and ADA vs PBO, respectively at week 16 (all P< 0.001; Figure 2); numerically greater reductions in total effect on pain were observed with UPA vs ADA. Direct and indirect effects on pain assessed as improvement in PtGA of pain were numerically greater with UPA vs ADA (Figure 2). Improvement in elicited pain assessed asTJC28 was also significantly greater with UPA and ADA vs PBO (all P< 0.05; Figure 3). Furthermore, UPA reduced pain assessed as TJC28 numerically more than ADA when examining the total effect explained by direct effects and the inflammatory mediators (indirect effects).
Conclusion: UPA and ADA produced relevantly higher mean improvements in pain via inflammatory or non-inflammatory mechanisms vs PBO in patients with PsA. Total effects on pain improvement were numerically greater with UPA vs ADA when assessed as PtGA of pain and TJC28.
Reference: 1Preacher, KJ & Hayes, AF. Behavior Research Methods,2008;40(3), 879–891.

Figure 1. Change from Baseline in PtGA of Pain (mm) at Week 16

Figure 2. Direct and Indirect Effects of Treatment on Pain Assessed as Improvement in PtGA of Pain at Week 16

Figure 3. Direct and Indirect Effects of Treatment on Pain Assessed as Improvement in TJC28 at Week 16
P. Taylor: AbbVie, 2, Biogen, 2, Eli Lilly, 2, Fresenius, 2, Galapagos, 2, 5, Gilead Sciences, 2, GSK, 2, Janssen, 2, Nordic Pharma, 2, Pfizer Inc, 2, Sanofi, 2, UCB, 2; D. Walsh: AbbVie, 2, Contura International A/S, 2, Eli Lilly, 5, GlaxoSmithKline Research & Development Limited, 5, Orion Corporation, 5, Pfizer, 5; T. Takeuchi: AbbVie, 2, 5, 6, AYUMI, 5, Bristol-Myers Squibb, 6, Chugai, 2, 5, 6, Daiichi Sankyo, 5, Eisai, 5, 6, Eli Lilly Japan, 2, 6, Gilead, 2, 6, Janssen, 6, Mitsubishi-Tanabe, 2, 5, 6, ONO, 5, Pfizer Japan, 6, Taiho, 2; B. Fautrel: AbbVie, 2, BMS, 2, Chugai, 2, Fresenius Kabi, 2, Galapagos, 2, Lilly, 2, Medac, 2, Nordic Pharma, 2, Novartis, 2, Pfizer, 2, Sobi, 2, UCB, 2; J. Pope: AbbVie, 1, 2; K. Kato: AbbVie/Abbott, 3, 11; A. Setty: AbbVie, 3, 11; T. Gao: AbbVie, 3, 11; D. Caballero: AbbVie, 3, 11; R. Lippe: AbbVie, 3, 11; A. Kavanaugh: AbbVie, 1, 2, Amgen, 1, 2, BMS, 1, 2, Eli Lilly, 1, 2, Novartis, 1, 2, Pfizer, 1, 2, UCB, 1, 2.



