Poster Session C
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (2195–2226) Spondyloarthritis Including Psoriatic Arthritis – Diagnosis, Manifestations, & Outcomes Poster III: SpA
2215: Can Axial Spondyloarthritis Unequivocally Be Diagnosed by Rheumatologists in Patients with Chronic Back Pain of Less Than Two Years Duration? The Primary Outcome of the Two-year SPondyloArthritis Caught Early (SPACE) Cohort
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall

Mary Lucy Marques, MD, MSc
Leiden University Medical Center
Coimbra, PortugalDisclosure information not submitted.
Abstract Poster Presenter(s)
Mary Lucy marques1, Sofia Ramiro2, Miranda Van Lunteren3, Rosalinde Stal3, Robert BM Landewé4, Marleen van de Sande5, Karen Minde Fagerli6, Inger Jorid Berg6, Maikel van oosterhout7, Sofia Exarchou8, Roberta Ramonda9, Désirée van der Heijde2 and Floris Van Gaalen3, 1Leiden University Medical Center; Centro Hospitalar e Universitário de Coimbra (Department of Rheumatology), Coimbra, Portugal, 2Department of Rheumatology, Leiden University Medical Center, Leiden, Netherlands, 3Leiden University Medical Center, Department of Rheumatology, Leiden, Netherlands, 4Amsterdam Rheumatology & Clinical Immunology Center, Amsterdam and Zuyderland MC, Herleen, Netherlands, 5Amsterdam UMC, University of Amsterdam, Department of Rheumatology & Clinical Immunology and Department of Experimental Immunology, Amsterdam Infection & Immunity Institute; Amsterdam Rheumatology & Immunology Center (ARC), Academic Medical Center, Amsterdam, Netherlands, 6Center for Treatment of Rheumatic and Musculoskeletal Diseases (REMEDY), Diakonhjemmet Hospital, Oslo, Norway, 7Groene Hart Ziekenhuis, Gouda, Netherlands, 8Lund University, Åkarp, Sweden, 9University of Padova, Department of Rheumatology, Padova, Italy
Background/Purpose: Unacceptable diagnostic delay in axial Spondyloarthritis (axSpA) remains an issue. In 2008, the longitudinal SPondyloArthritis Caught Early (SPACE)-cohort started to assess the prevalence of axSpA and the reliability of an early diagnosis in patients with chronic back (CBP) of unknown origin. Here we present the primary outcomes of SPACE. We aimed to assess the two-year (2y) prevalence of an axSpA diagnosis in patients with recent onset CBP referred to the rheumatologist; the sustainability of a baseline (BL) diagnosis of axSpA when reviewed after 2y; and to explore BL patient differences of those with and without an axSpA diagnosis at 2y.
Methods: We analysed the 2y data from SPACE, a European inception cohort of patients (< 45y) with CBP of recent onset (≥3 months, ≤2y) and unknown origin. The full diagnostic work-up included clinical SpA features, acute phase reactants, HLA-B27, radiographs and MRI of the sacroiliac joints (SI-CR and SI-MRI) and spine (data not shown). Patients with an increased likelihood of having axSpA (≥1 major or ≥2 minor prespecified SpA features) were eligible for follow-up. The clinical diagnosis at 2y was the main outcome of this study. At each visit, the treating rheumatologist judged on the presence or absence of axSpA (axSpA or no-axSpA) with a level of confidence (LoC) on a numeric rating scale (0: not confident at all to 10: very confident). The main outcome was the presence of 'definite axSpA' at 2y, defined by a clinical diagnosis of axSpA with LoC ≥7 at 2y (complete follow-up) or at the two last available visits (missing at 2y). 'No axSpA' was defined as not having axSpA at 2y (LoC ≥7; or if LoC < 7, plus an alternative diagnosis for CBP reported). All other patients were considered to have 'uncertain' diagnosis (Figure 1). ASAS classification criteria were computed using sacroiliitis local reading in definite axSpA patients. We assessed the prevalence of definite axSpA at 2y as well as changes in diagnosis over time, and descriptively summarised BL characteristics.
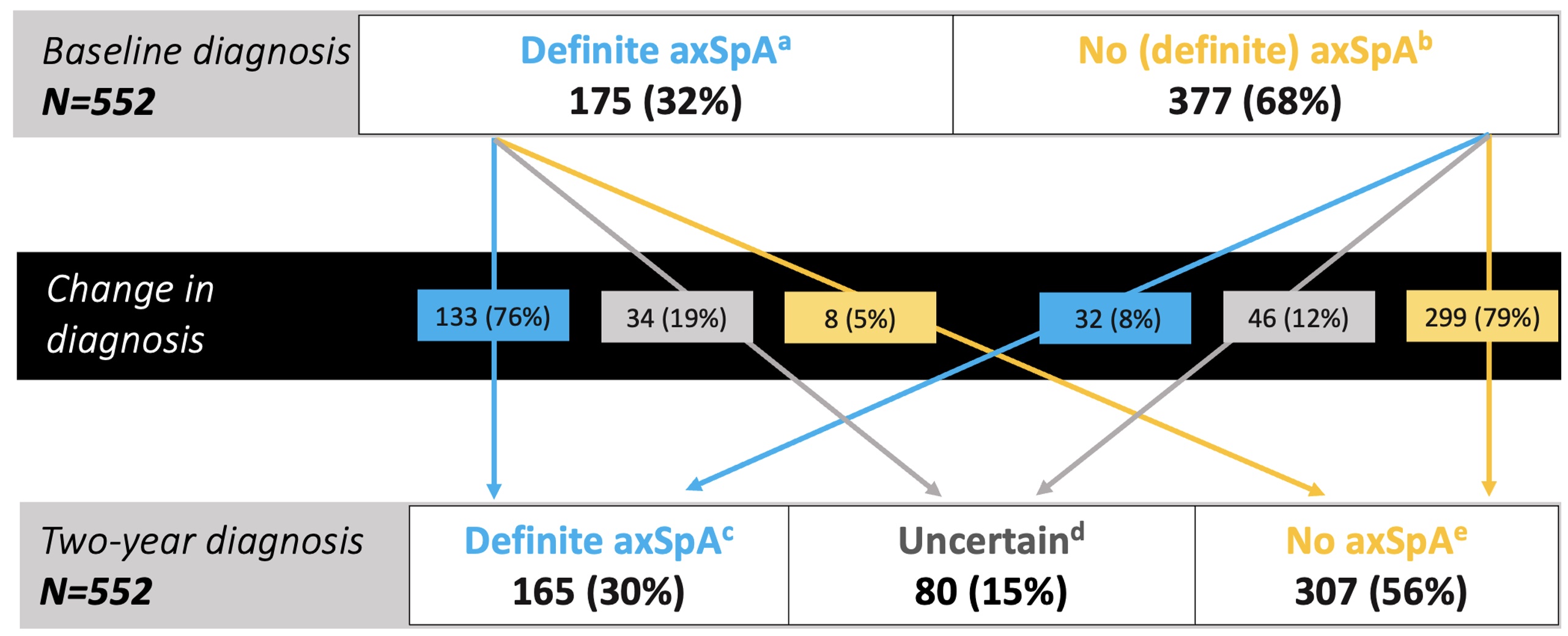
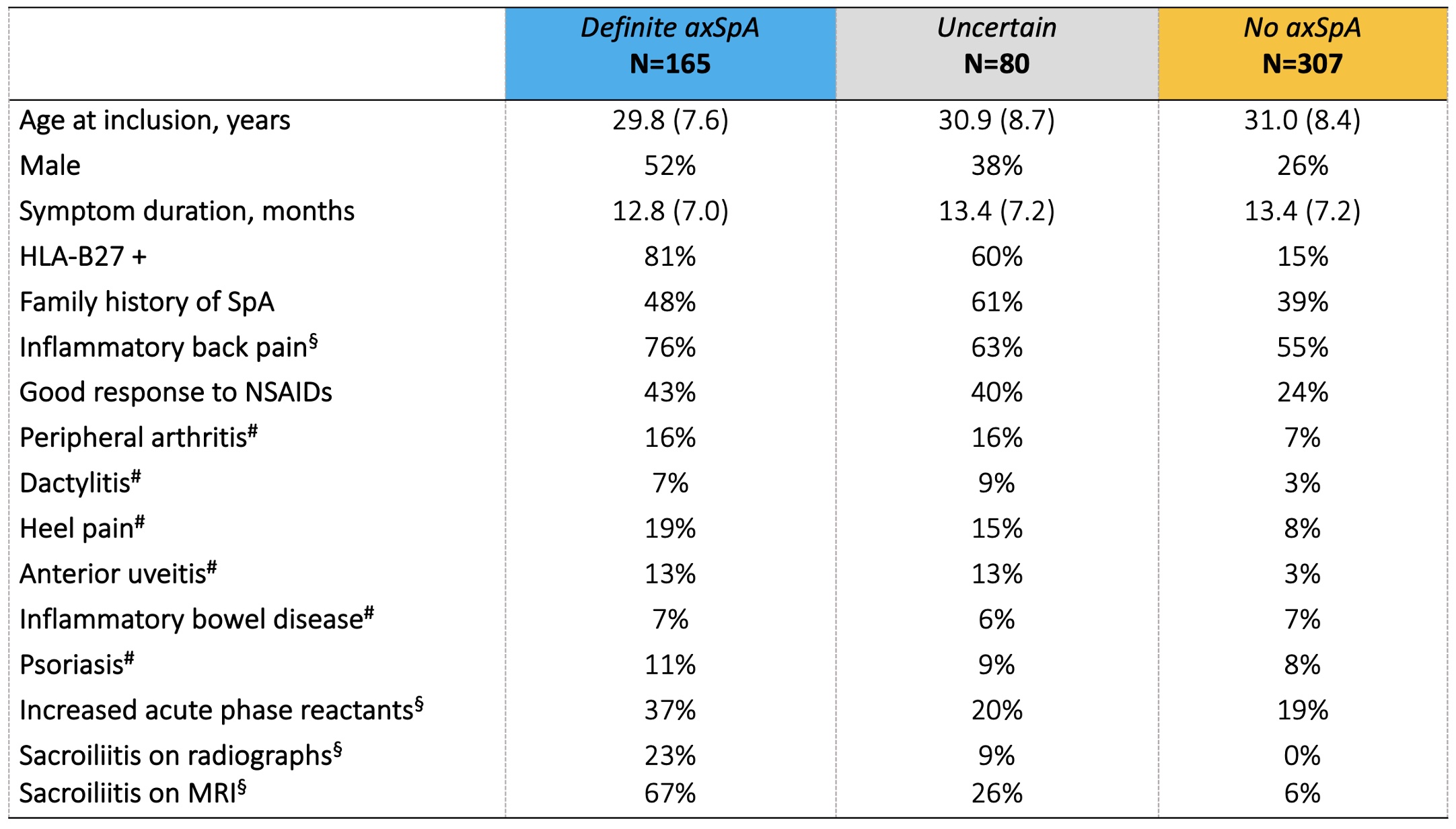
Results: We included 552 CBP patients (Leiden n=383, Oslo n=94, Amsterdam n=48, and Gouda n=27).A diagnosis of definite axSpA was given to 175 (32%) patients at BL and 165 (30%) at 2y (Figure 1). The mean (SD) LoC were 8.1 (2.0) and 8.7 (1.0), with 155/175 (89%) and 145/165 (87%) fulfilling ASAS classification criteria, respectively. BL diagnostic judgments were relatively unequivocal and remained rather stable: At 2y, 5% of the BL diagnoses of definite axSpA were refuted; and -vice versa: 8% of those who did not obtain a BL diagnosis of axSpA 'gained' one at 2y. Diagnostic uncertainty remained in 15% of CBP-patients. Expectedly, BL SpA features were more prevalent in the 2y definite axSpA group (Table 1). HLA-B27 status and (presence or absence of) imaging-detected sacroiliitis at BL appeared the best discriminator(s) between definite axSpA and no axSpA at 2y.
Conclusion: One third of patients with CBP of recent onset referred to the rheumatologist has definite axSpA. Most patients can be unequivocally and reliably diagnosed at first assessment, though residual diagnostic uncertainty persisted after 2y. None of the many SpA features suffices alone, but HLA-B27 positivity andsacroiliitis on imaging best discriminate the 2y diagnostic groups.
M. marques: None; S. Ramiro: AbbVie, 2, 5, Eli Lilly, 2, Galapagos, 5, MSD, 2, 5, Novartis, 2, 5, Pfizer, 2, 5, Sanofi, 2, UCB Pharma, 2, 5; M. Van Lunteren: None; R. Stal: None; R. Landewé: AbbVie, 2, 5, AstraZeneca, 2, BMS, 2, Eli Lilly, 2, Novartis, 2, 5, Pfizer, 2, 5, Rheumatology Consultancy BV, 12, Owner, UCB Pharma, 2, 5; M. van de Sande: AbbVie, 2, Eli Lilly, 5, Janssen, 6, Novartis, 2, 5, 6, UCB Pharma, 2, 5, 6; K. Minde Fagerli: None; I. Jorid Berg: None; M. van oosterhout: None; S. Exarchou: Amgen, 2, Janssen, 2, Novartis, 2, UCB Pharma, 2; R. Ramonda: None; D. van der Heijde: AbbVie, 2, Bayer, 2, BMS, 2, Eli Lilly, 2, Galapagos, 2, Gilead, 2, GSK, 2, Imaging Rheumatology BV, 12, Director, Janssen, 2, Novartis, 2, Pfizer, 2, Takeda, 2, UCB Pharma, 2; F. Van Gaalen: AbbVie, 12, Personal fees, BMS, 12, Personal fees, Eli Lilly, 12, Personal fees, Jacobus Stichting, 5, MSD, 12, Personal fees, Novartis, 5, 12, Fees, Stichting ASAS, 5, Stichting Vrienden van Sole Mio, 5, UCB Pharma, 5.
Background/Purpose: Unacceptable diagnostic delay in axial Spondyloarthritis (axSpA) remains an issue. In 2008, the longitudinal SPondyloArthritis Caught Early (SPACE)-cohort started to assess the prevalence of axSpA and the reliability of an early diagnosis in patients with chronic back (CBP) of unknown origin. Here we present the primary outcomes of SPACE. We aimed to assess the two-year (2y) prevalence of an axSpA diagnosis in patients with recent onset CBP referred to the rheumatologist; the sustainability of a baseline (BL) diagnosis of axSpA when reviewed after 2y; and to explore BL patient differences of those with and without an axSpA diagnosis at 2y.
Methods: We analysed the 2y data from SPACE, a European inception cohort of patients (< 45y) with CBP of recent onset (≥3 months, ≤2y) and unknown origin. The full diagnostic work-up included clinical SpA features, acute phase reactants, HLA-B27, radiographs and MRI of the sacroiliac joints (SI-CR and SI-MRI) and spine (data not shown). Patients with an increased likelihood of having axSpA (≥1 major or ≥2 minor prespecified SpA features) were eligible for follow-up. The clinical diagnosis at 2y was the main outcome of this study. At each visit, the treating rheumatologist judged on the presence or absence of axSpA (axSpA or no-axSpA) with a level of confidence (LoC) on a numeric rating scale (0: not confident at all to 10: very confident). The main outcome was the presence of 'definite axSpA' at 2y, defined by a clinical diagnosis of axSpA with LoC ≥7 at 2y (complete follow-up) or at the two last available visits (missing at 2y). 'No axSpA' was defined as not having axSpA at 2y (LoC ≥7; or if LoC < 7, plus an alternative diagnosis for CBP reported). All other patients were considered to have 'uncertain' diagnosis (Figure 1). ASAS classification criteria were computed using sacroiliitis local reading in definite axSpA patients. We assessed the prevalence of definite axSpA at 2y as well as changes in diagnosis over time, and descriptively summarised BL characteristics.
Results: We included 552 CBP patients (Leiden n=383, Oslo n=94, Amsterdam n=48, and Gouda n=27).A diagnosis of definite axSpA was given to 175 (32%) patients at BL and 165 (30%) at 2y (Figure 1). The mean (SD) LoC were 8.1 (2.0) and 8.7 (1.0), with 155/175 (89%) and 145/165 (87%) fulfilling ASAS classification criteria, respectively. BL diagnostic judgments were relatively unequivocal and remained rather stable: At 2y, 5% of the BL diagnoses of definite axSpA were refuted; and -vice versa: 8% of those who did not obtain a BL diagnosis of axSpA 'gained' one at 2y. Diagnostic uncertainty remained in 15% of CBP-patients. Expectedly, BL SpA features were more prevalent in the 2y definite axSpA group (Table 1). HLA-B27 status and (presence or absence of) imaging-detected sacroiliitis at BL appeared the best discriminator(s) between definite axSpA and no axSpA at 2y.
Conclusion: One third of patients with CBP of recent onset referred to the rheumatologist has definite axSpA. Most patients can be unequivocally and reliably diagnosed at first assessment, though residual diagnostic uncertainty persisted after 2y. None of the many SpA features suffices alone, but HLA-B27 positivity andsacroiliitis on imaging best discriminate the 2y diagnostic groups.

Figure 1. Diagnosis course from baseline (BL) to the two-year (2y) anchor visit.
a. patients diagnosed at BL with axSpA with a Level of Confidence (LoC) ≥7; b. patients diagnosed at BL with axSpA (LoC <7) or with no axSpA (any LoC); c. patients diagnosed with axSpA with LoC ≥7 at 2y (if complete follow-up) or at the last two available visits (if missing the 2y visit); d. patients diagnosed with axSpA with LoC <7 at 2y (or LoC ≥7, if only baseline observation available), and patients with axSpA with LoC <7 at the last available observation and no consistent diagnosis at the last two observations nor alternative no axSpA diagnosis given at the last observation; e. patients with no axSpA at the last observation over 2y with LoC ≥7 (or if <7, plus an alternative no axSpA diagnosis reported, e.g. fibromyalgia or aspecific back pain). axSpA: axial Spondyloarthritis.
a. patients diagnosed at BL with axSpA with a Level of Confidence (LoC) ≥7; b. patients diagnosed at BL with axSpA (LoC <7) or with no axSpA (any LoC); c. patients diagnosed with axSpA with LoC ≥7 at 2y (if complete follow-up) or at the last two available visits (if missing the 2y visit); d. patients diagnosed with axSpA with LoC <7 at 2y (or LoC ≥7, if only baseline observation available), and patients with axSpA with LoC <7 at the last available observation and no consistent diagnosis at the last two observations nor alternative no axSpA diagnosis given at the last observation; e. patients with no axSpA at the last observation over 2y with LoC ≥7 (or if <7, plus an alternative no axSpA diagnosis reported, e.g. fibromyalgia or aspecific back pain). axSpA: axial Spondyloarthritis.

Table 1. Baseline characteristics by two-year (2y) diagnosis of patients with chronic back pain duration of ≥3 months but ≤2y, < 45y of age.
# Currently present or past presence (if confirmed/reported by a physician). § Local data. axSpA: axial Spondyloarthritis; MRI: magnetic resonance imaging.
# Currently present or past presence (if confirmed/reported by a physician). § Local data. axSpA: axial Spondyloarthritis; MRI: magnetic resonance imaging.
M. marques: None; S. Ramiro: AbbVie, 2, 5, Eli Lilly, 2, Galapagos, 5, MSD, 2, 5, Novartis, 2, 5, Pfizer, 2, 5, Sanofi, 2, UCB Pharma, 2, 5; M. Van Lunteren: None; R. Stal: None; R. Landewé: AbbVie, 2, 5, AstraZeneca, 2, BMS, 2, Eli Lilly, 2, Novartis, 2, 5, Pfizer, 2, 5, Rheumatology Consultancy BV, 12, Owner, UCB Pharma, 2, 5; M. van de Sande: AbbVie, 2, Eli Lilly, 5, Janssen, 6, Novartis, 2, 5, 6, UCB Pharma, 2, 5, 6; K. Minde Fagerli: None; I. Jorid Berg: None; M. van oosterhout: None; S. Exarchou: Amgen, 2, Janssen, 2, Novartis, 2, UCB Pharma, 2; R. Ramonda: None; D. van der Heijde: AbbVie, 2, Bayer, 2, BMS, 2, Eli Lilly, 2, Galapagos, 2, Gilead, 2, GSK, 2, Imaging Rheumatology BV, 12, Director, Janssen, 2, Novartis, 2, Pfizer, 2, Takeda, 2, UCB Pharma, 2; F. Van Gaalen: AbbVie, 12, Personal fees, BMS, 12, Personal fees, Eli Lilly, 12, Personal fees, Jacobus Stichting, 5, MSD, 12, Personal fees, Novartis, 5, 12, Fees, Stichting ASAS, 5, Stichting Vrienden van Sole Mio, 5, UCB Pharma, 5.



