Poster Session C
Imaging
Session: (1862–1894) Imaging of Rheumatic Diseases Poster II
1882: Imaging in Diagnosis, Monitoring and Outcome Prediction of Large Vessel Vasculitis: A Systematic Literature Review Informing the 2023 Update of the EULAR Recommendations
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall

Philipp Bosch, MD, MSc
Medical University of Graz
Graz, AustriaDisclosure information not submitted.
Abstract Poster Presenter(s)
Philipp Bosch1, Milena Bond2, Christian Dejaco3, Cristina Ponte4, Sarah Mackie5, Louise Falzon6, Wolfgang Schmidt7 and Sofia Ramiro8, 1Medical University of Graz, Graz, Austria, 2Azienda sanitaria dell'Alto Adige, Merano, Italy, 3Department of Rheumatology, Medical University Graz, Graz, Austria; Department of Rheumatology, Hospital of Bruneck (ASAA-SABES), Teaching Hospital of the Paracelsius Medical University, Brunico, Italy, 4Department of Rheumatology, Centro Hospitalar Universitário Lisboa Norte, Centro Académico de Medicina de Lisboa, Lisbon, Portugal; Rheumatology Research Unit, Instituto de Medicina Molecular, Faculdade de Medicina, Universidade de Lisboa, Centro Académico de Medicina de Lisboa, Lisbon, Portugal, 5Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, Leeds, UK; Leeds Biomedical Research Centre, Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom, 6University of Sheffield, Sheffield, United Kingdom, 7Immanuel Krankenhaus Berlin, Berlin, Germany, 8Department of Rheumatology, Leiden University Medical Center, Leiden, Netherlands
Background/Purpose: Since the development of the EULAR recommendations for the use of imaging in large vessel vasculitis (LVV) in 2017, new data has emerged in the field of imaging techniques and their application in the diagnosis and follow-up of patients with giant cell arteritis (GCA) and Takayasu arteritis (TAK).The objective of this review was to summarize the evidence on different imaging techniques for diagnosis, monitoring, and outcome prediction in LVV in order to inform a EULAR task force updating the recommendations for imaging in LVV.
Methods: Systematic literature review (SLR) on studies published between 2017-2022 on ultrasound (US), magnetic resonance imaging (MRI), computed tomography (CT), positron emission tomography (PET)-CT/MRI and fluorescein angiography in patients with LVV (PROSPERO registration CRD42022360545). Eligible study designs included randomized controlled trials and observational studies but excluded case-controlled studies. Two reviewers independently performed data extraction, synthesis, and risk of bias assessment. For studies on diagnosis, meta-analyses were performed using data from both the original and updated SLR whenever possible. Pooled sensitivities and specificities were obtained by fitting random effects models for all studies and for studies with low risk of bias separately. Meta-analyses were performed in R version 4.2.1. using the "lme4" package. The description of observations without inferences and the heterogeneity of reported data precluded any meta-analysis for outcome prediction or monitoring.
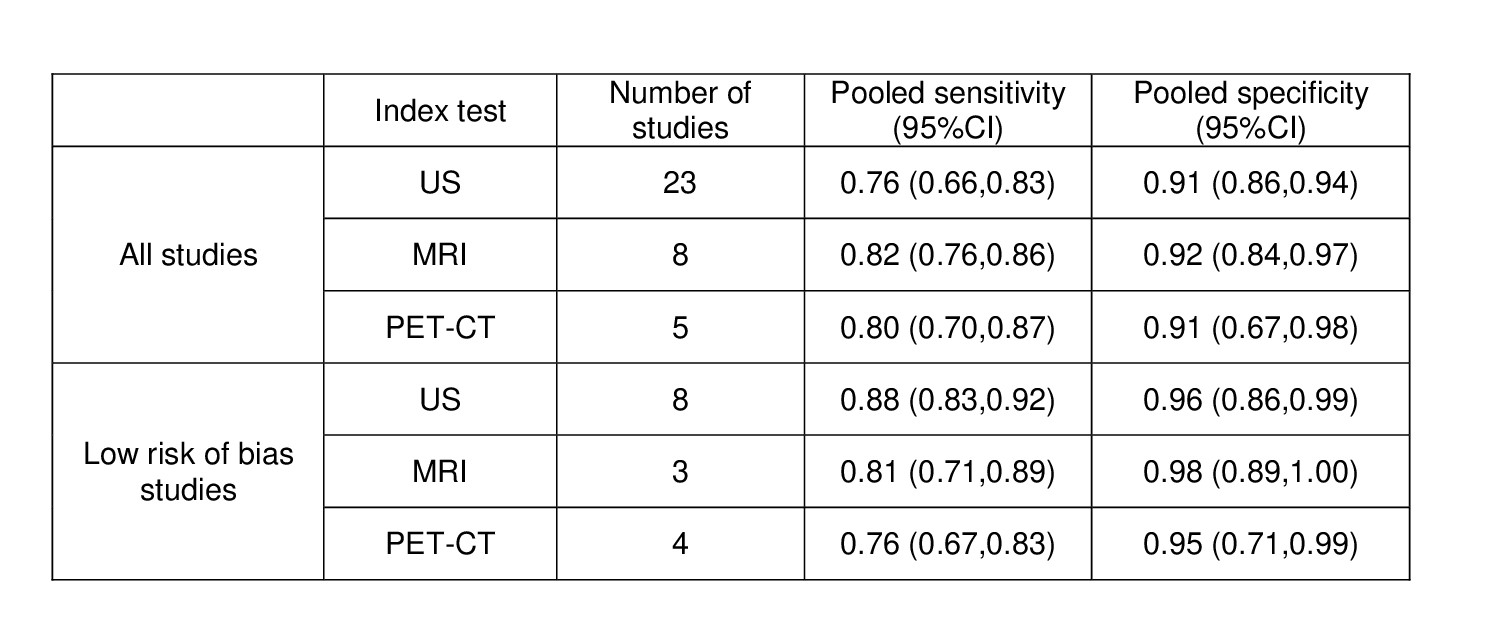
Results: A total of 4696 references were identified. Thirty-eight studies on GCA (n=32), TAK (n=2), and GCA and TAK (n=4) were included through the update, adding up to eighty-one studies from both SLRs. Pooled sensitivities and specificities for US, MRI and PET-CT using a clinical diagnosis of GCA as the reference standard are depicted in table 1. No studies on the diagnostic value of imaging techniques were found for TAK. The US evaluation of patients with suspected GCA, including the assessment of both cranial and extracranial vessels, showed a higher pooled sensitivity (95%CI) (89% [73%-96%] vs 70% [59%-79%]) and similar specificity (95%CI) (91% [83%-95%] vs 91% [84%-94%]) compared to only including cranial vessels. Studies on outcome prediction (n=5) and monitoring (n=10) reported change of signs of vasculitis along with disease activity and proposed composite scores comprising several vessel territories for US, MRI and PET-CT.
Conclusion: US, MRI and PET-CT revealed a good performance for the diagnosis of GCA. Assessing both cranial and extracranial vessels with US leads to a higher pooled sensitivity with a similar pooled specificity compared to an assessment limited to cranial vessels.
P. Bosch: Janssen, 6, 12, Congress fees, Pfizer, 5; M. Bond: AbbVie/Abbott, 5; C. Dejaco: AbbVie/Abbott, 1, 5, 6, Eli Lilly, 6, Galapagos Pharma, 1, 6, Janssen, 1, 6, Novartis, 1, 6, Pfizer, 6, Sparrow, 1; C. Ponte: None; S. Mackie: AbbVie/Abbott, 2, AstraZeneca, 2, GlaxoSmithKlein(GSK), 3, 12, Investigator, National Institute for Health and Care Research, 5, 12, investigator on STERLING-PMR trial, funded by NIHR; patron of the charity PMRGCAuk, Pfizer, 2, 6, Roche, 2, 6, 12, Support from Roche/Chugai to attend EULAR2019 in person, Sanofi, 2, 12, Investigator, Sparrow, 12, Investigator, UCB and Novartis, 6, Vifor, 6; L. Falzon: None; W. Schmidt: AbbVie/Abbott, 2, 5, 6, Amgen, 2, 6, Bristol-Myers Squibb(BMS), 2, 6, Chugai, 2, 6, Eli Lilly, 2, GlaxoSmithKlein(GSK), 5, 6, Johnson & Johnson, 2, Medac, 2, Novartis, 2, 5, 6, Pfizer, 2, Roche, 2, 6, Sanofi, 2, 5, 6, UCB, 2; S. Ramiro: AbbVie, 2, 5, Eli Lilly, 2, Galapagos, 5, MSD, 2, 5, Novartis, 2, 5, Pfizer, 2, 5, Sanofi, 2, UCB Pharma, 2, 5.
Background/Purpose: Since the development of the EULAR recommendations for the use of imaging in large vessel vasculitis (LVV) in 2017, new data has emerged in the field of imaging techniques and their application in the diagnosis and follow-up of patients with giant cell arteritis (GCA) and Takayasu arteritis (TAK).The objective of this review was to summarize the evidence on different imaging techniques for diagnosis, monitoring, and outcome prediction in LVV in order to inform a EULAR task force updating the recommendations for imaging in LVV.
Methods: Systematic literature review (SLR) on studies published between 2017-2022 on ultrasound (US), magnetic resonance imaging (MRI), computed tomography (CT), positron emission tomography (PET)-CT/MRI and fluorescein angiography in patients with LVV (PROSPERO registration CRD42022360545). Eligible study designs included randomized controlled trials and observational studies but excluded case-controlled studies. Two reviewers independently performed data extraction, synthesis, and risk of bias assessment. For studies on diagnosis, meta-analyses were performed using data from both the original and updated SLR whenever possible. Pooled sensitivities and specificities were obtained by fitting random effects models for all studies and for studies with low risk of bias separately. Meta-analyses were performed in R version 4.2.1. using the "lme4" package. The description of observations without inferences and the heterogeneity of reported data precluded any meta-analysis for outcome prediction or monitoring.
Results: A total of 4696 references were identified. Thirty-eight studies on GCA (n=32), TAK (n=2), and GCA and TAK (n=4) were included through the update, adding up to eighty-one studies from both SLRs. Pooled sensitivities and specificities for US, MRI and PET-CT using a clinical diagnosis of GCA as the reference standard are depicted in table 1. No studies on the diagnostic value of imaging techniques were found for TAK. The US evaluation of patients with suspected GCA, including the assessment of both cranial and extracranial vessels, showed a higher pooled sensitivity (95%CI) (89% [73%-96%] vs 70% [59%-79%]) and similar specificity (95%CI) (91% [83%-95%] vs 91% [84%-94%]) compared to only including cranial vessels. Studies on outcome prediction (n=5) and monitoring (n=10) reported change of signs of vasculitis along with disease activity and proposed composite scores comprising several vessel territories for US, MRI and PET-CT.
Conclusion: US, MRI and PET-CT revealed a good performance for the diagnosis of GCA. Assessing both cranial and extracranial vessels with US leads to a higher pooled sensitivity with a similar pooled specificity compared to an assessment limited to cranial vessels.

Table 1. Pooled sensitivities and specificities of diagnostic studies on GCA with clinical diagnosis as reference standard
GCA, giant cell arteritis; MRI, magnetic resonance imaging; PET-CT, positron emission tomography – computed tomography; US, ultrasound
GCA, giant cell arteritis; MRI, magnetic resonance imaging; PET-CT, positron emission tomography – computed tomography; US, ultrasound
P. Bosch: Janssen, 6, 12, Congress fees, Pfizer, 5; M. Bond: AbbVie/Abbott, 5; C. Dejaco: AbbVie/Abbott, 1, 5, 6, Eli Lilly, 6, Galapagos Pharma, 1, 6, Janssen, 1, 6, Novartis, 1, 6, Pfizer, 6, Sparrow, 1; C. Ponte: None; S. Mackie: AbbVie/Abbott, 2, AstraZeneca, 2, GlaxoSmithKlein(GSK), 3, 12, Investigator, National Institute for Health and Care Research, 5, 12, investigator on STERLING-PMR trial, funded by NIHR; patron of the charity PMRGCAuk, Pfizer, 2, 6, Roche, 2, 6, 12, Support from Roche/Chugai to attend EULAR2019 in person, Sanofi, 2, 12, Investigator, Sparrow, 12, Investigator, UCB and Novartis, 6, Vifor, 6; L. Falzon: None; W. Schmidt: AbbVie/Abbott, 2, 5, 6, Amgen, 2, 6, Bristol-Myers Squibb(BMS), 2, 6, Chugai, 2, 6, Eli Lilly, 2, GlaxoSmithKlein(GSK), 5, 6, Johnson & Johnson, 2, Medac, 2, Novartis, 2, 5, 6, Pfizer, 2, Roche, 2, 6, Sanofi, 2, 5, 6, UCB, 2; S. Ramiro: AbbVie, 2, 5, Eli Lilly, 2, Galapagos, 5, MSD, 2, 5, Novartis, 2, 5, Pfizer, 2, 5, Sanofi, 2, UCB Pharma, 2, 5.



