Poster Session C
Rheumatoid arthritis (RA)
Session: (1734–1775) RA – Etiology and Pathogenesis Poster
1752: Fibroblast Expression of Neurotransmitter Receptor HTR2A Associates with Inflammation in Rheumatoid Arthritis Joint
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- CX
Chunyan Xiang, -None-
Shanghai Institute of Rheumatology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University (SJTUSM)
shanghai, Shanghai, ChinaDisclosure information not submitted.
Abstract Poster Presenter(s)
Chunyan Xiang1, Soomin Hong1, Bingjiao Zhao2, Hui Pi3, Fang Du4, Xingyu Lu5, Yuanjia Tang1, Nan Shen6, Chunxi Yang7 and Runci Wang8, 1Shanghai Institute of Rheumatology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University (SJTUSM), Shanghai, China, 2Department of Orthodontics, Shanghai Stomatological Hospital & School of Stomatology, Fudan University, Shanghai, China, 3Jiangxi Provincial People's Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, China, 4Department of Rheumatology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University (SJTUSM), Shanghai, China, 5Department of Endocrinology, Xinhua Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, 6Shanghai Jiao Tong University School of Medicine, Shanghai, China, 7Department of Orthopedics, Renji Hospital, School of Medicine, Shanghai Jiao Tong University (SJTUSM), Shanghai, China, 8Renji hospital, Shanghai Jiaotong University, Pudong Xinqu, China
Background/Purpose: Peripheral neuroimmune crosstalk plays a crucial role in the inflammatory process and bone metabolism in joint. Serotonin receptor HTR2A was reported to be expressed on immune cells, of which gene polymorphisms are associated with an increased risk of developing RA and other autoimmune disease. However, the expression and regulation of HTR2A in arthritis synovium remain poorly understood.
Methods: Differential expression of neurotransmitter receptors (NTRs) and their correlated inflammatory molecules was identified in RA and OA synovium from public scRNA-seq dataset[1]. IHC staining of synovial tissue from RA and OA patients was performed for validation. Expression of miRNAs potentially targeting HTR2A carried by synovial fluid extracellular vesicles (EVs) was screened in low- and high-grade inflammation RA from public dataset[2] and validated by qPCR.
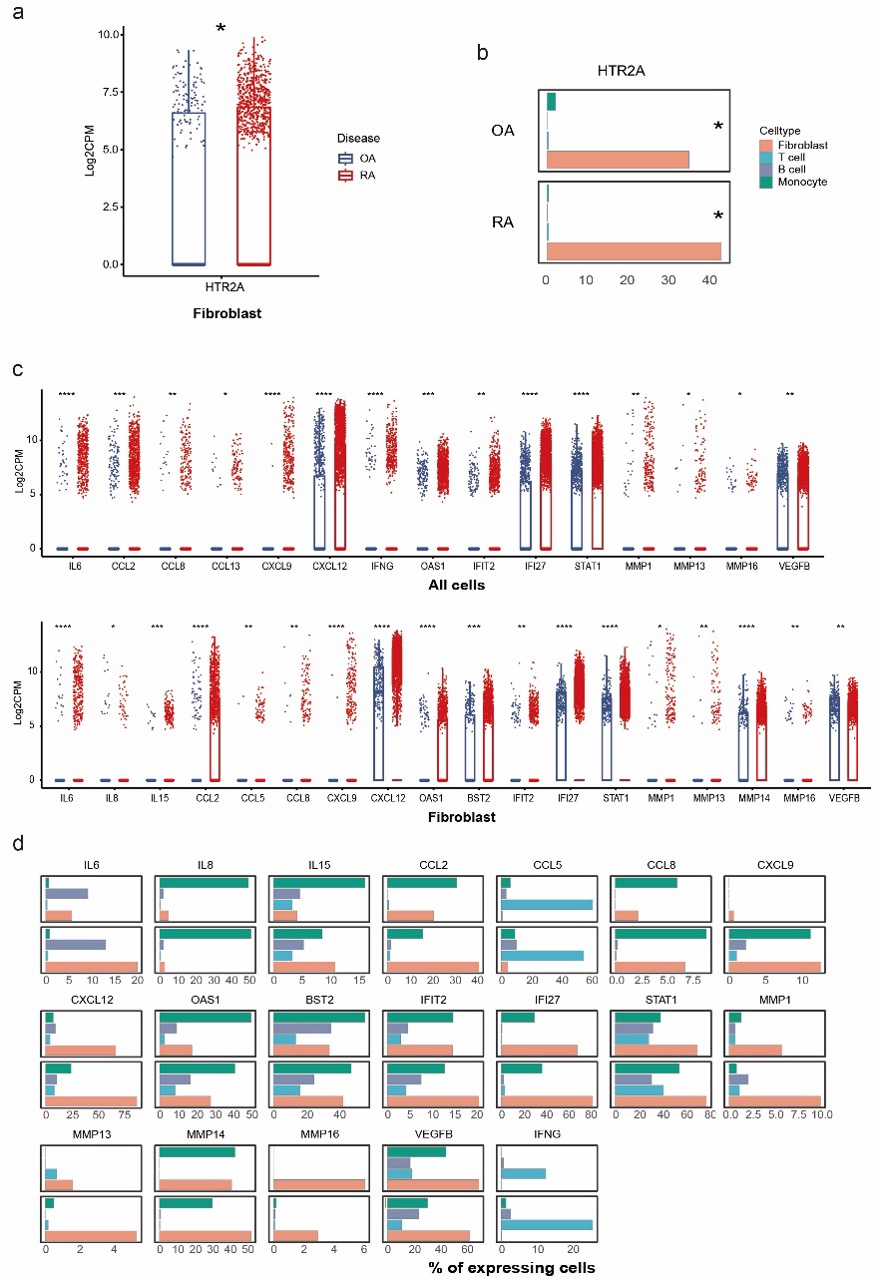
Results: HTR2A was expressed by 34.3% and 42.1% of fibroblasts in OA and RA synovium, while barely expressed by synovial leukocytes. Higher level of IL-6, IFN-𝛾, IFN stimulated genes, chemokines including CCL2, CCL8, CCL13, CXCL9, and CXCL12, and MMPs and VEGFB were observed on all cell types in RA joint, indicating a highly inflammatory microenvironment in RA. (Fig.1).
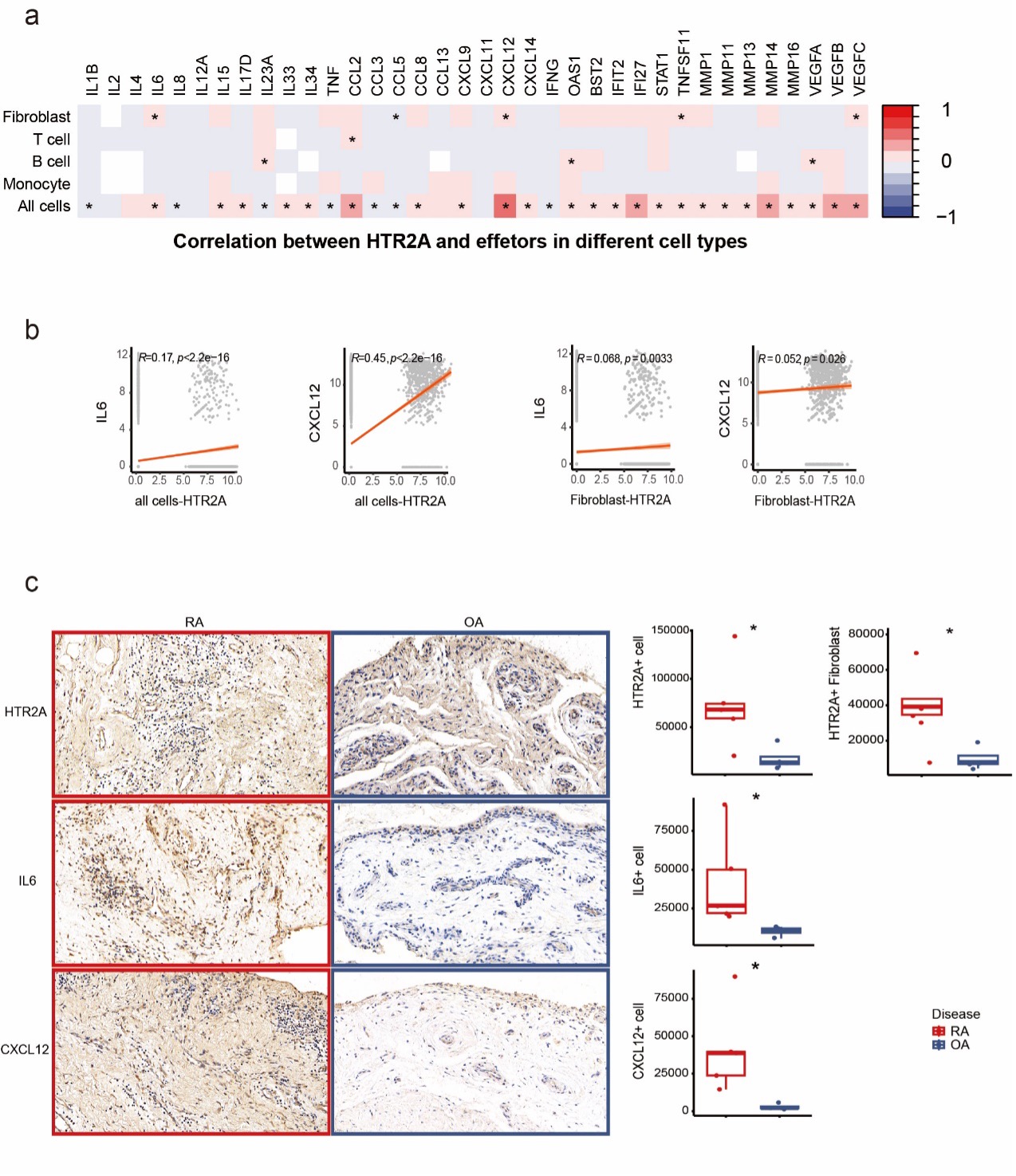
Correlation analysis showed positive correlation of HTR2A with IL6, CCL5, CXCL12, TNFSF11 and VEGFC expression in fibroblast specifically as well as in all synovial cells. The strongest correlation was found between HTR2A and CXCL12 (R=0.45). Transcriptomic findings above were confirmed on the protein level with IHC staining of RA and OA synovial tissue from patients. There was significantly upregulated expression of HTR2A on RA whole synovial tissue level and fibroblast level. Increased expression of IL-6 and CXCL12 was also found in RA synovial tissue (Fig.2).
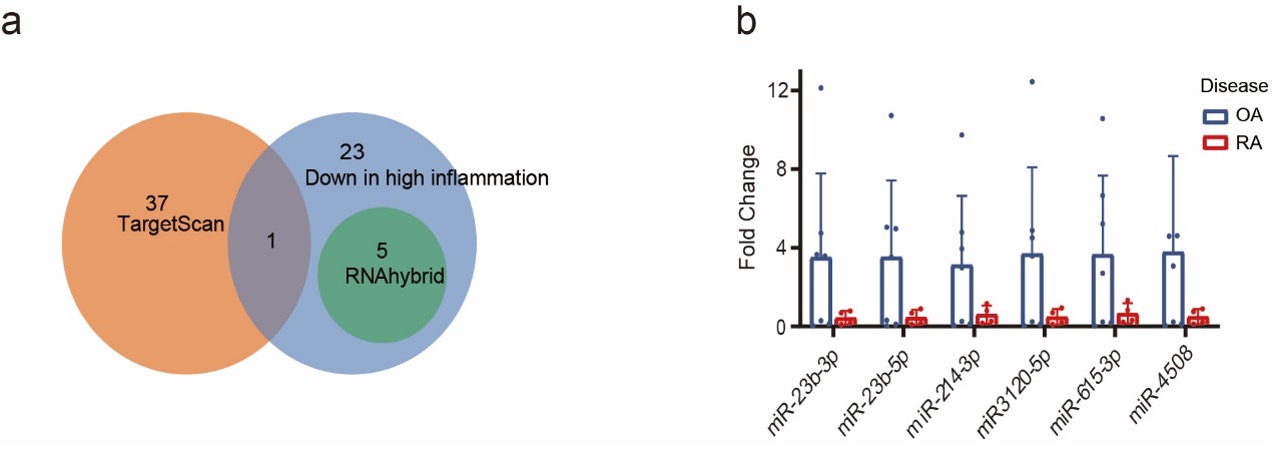
To investigate what could regulate the different HTR2A expression in RA and OA, we examined the content of EVs derived from RA and OA synovial fluid. Integrated with public RNAseq data, TargetScan and RNAhybrid prediction, 6 miRNAs predicted to regulate HTR2A expression, miRNA-23b-3p, miR-23b-5p, miR-214-3p, miR-3120-5P, miR-615-3p, and miR-4508, were significantly lower in RA EVs. A trend of lower expression of these 6 miRNAs was demonstrated by qPCR (Fig.3).
Conclusion: A neurotransmitter receptor of serotonin, HTR2A, was enriched in rheumatoid arthritis (RA) synovial fibroblast and positively correlated with inflammation. 6 miRNAs targeting HTR2A were decreased in RA synovial fluid EVs comparing to osteoarthritis. HTR2A may contribute to inflammation and RA pathogenesis, and miRNAs targeting HTR2A might offer new therapeutic strategies to alleviate inflammation in RA.
C. Xiang: None; S. Hong: None; B. Zhao: None; H. Pi: None; F. Du: None; X. Lu: None; Y. Tang: None; N. Shen: None; C. Yang: None; R. Wang: None.
Background/Purpose: Peripheral neuroimmune crosstalk plays a crucial role in the inflammatory process and bone metabolism in joint. Serotonin receptor HTR2A was reported to be expressed on immune cells, of which gene polymorphisms are associated with an increased risk of developing RA and other autoimmune disease. However, the expression and regulation of HTR2A in arthritis synovium remain poorly understood.
Methods: Differential expression of neurotransmitter receptors (NTRs) and their correlated inflammatory molecules was identified in RA and OA synovium from public scRNA-seq dataset[1]. IHC staining of synovial tissue from RA and OA patients was performed for validation. Expression of miRNAs potentially targeting HTR2A carried by synovial fluid extracellular vesicles (EVs) was screened in low- and high-grade inflammation RA from public dataset[2] and validated by qPCR.
Results: HTR2A was expressed by 34.3% and 42.1% of fibroblasts in OA and RA synovium, while barely expressed by synovial leukocytes. Higher level of IL-6, IFN-𝛾, IFN stimulated genes, chemokines including CCL2, CCL8, CCL13, CXCL9, and CXCL12, and MMPs and VEGFB were observed on all cell types in RA joint, indicating a highly inflammatory microenvironment in RA. (Fig.1).
Correlation analysis showed positive correlation of HTR2A with IL6, CCL5, CXCL12, TNFSF11 and VEGFC expression in fibroblast specifically as well as in all synovial cells. The strongest correlation was found between HTR2A and CXCL12 (R=0.45). Transcriptomic findings above were confirmed on the protein level with IHC staining of RA and OA synovial tissue from patients. There was significantly upregulated expression of HTR2A on RA whole synovial tissue level and fibroblast level. Increased expression of IL-6 and CXCL12 was also found in RA synovial tissue (Fig.2).
To investigate what could regulate the different HTR2A expression in RA and OA, we examined the content of EVs derived from RA and OA synovial fluid. Integrated with public RNAseq data, TargetScan and RNAhybrid prediction, 6 miRNAs predicted to regulate HTR2A expression, miRNA-23b-3p, miR-23b-5p, miR-214-3p, miR-3120-5P, miR-615-3p, and miR-4508, were significantly lower in RA EVs. A trend of lower expression of these 6 miRNAs was demonstrated by qPCR (Fig.3).
Conclusion: A neurotransmitter receptor of serotonin, HTR2A, was enriched in rheumatoid arthritis (RA) synovial fibroblast and positively correlated with inflammation. 6 miRNAs targeting HTR2A were decreased in RA synovial fluid EVs comparing to osteoarthritis. HTR2A may contribute to inflammation and RA pathogenesis, and miRNAs targeting HTR2A might offer new therapeutic strategies to alleviate inflammation in RA.

Figure 1. HTR2A and effectors expression pattern in joint synovial tissue of RA and OA patients. (a) Expression level and (b) positive percentage of HTR2A in RA and OA synovial fibroblast, (c) expression level and (d) positive percentage of inflammatory molecules in RA and OA synovium. Statistical test by Mann–Whitney U test in (a) and (c), *p < 0.05, **p < 0.01, ***p < 0.001,****p < 0.0001. Statistical test by Chi-square or Fisher's exact test in (b) and (d), *p < 0.05.

Figure 2. Inflammatory correlation and miRNA regulation of HTR2A in arthritis joints. (a) Heatmap showing correlation between HTR2A and inflammatory molecules using Pearson correlation. (b) Significant correlation plot between HTR2A and representative inflammatory molecules in fibroblast or all synovial cells. (c) 40x field view and quantification of immunohistochemical staining of HTR2A, IL6 and CXCL12 in synovial tissue of RA (n=5) and OA (n=4) patients, p values were calculated by Mann–Whitney U test, *p < 0.05, **p < 0.01, ***p < 0.001,****p < 0.0001.

Figure 3. miRNAs targeting HTR2A carried by synovial fluid EVs. (a) Venn diagram of miRNAs targeting HTR2A. miRNAs predicted by TargetScan (orange) and RNAhybrid (green) databases, and miRNAs lowly expressed in high-inflammation RA synovial fluid EVs (blue). (b) miRNAs were extracted from OA(n=7) and RA(n=4) synovial fluid EVs, and the expression of 6 miRNAs targeting for HTR2A were validated by qPCR. p values were calculated by t-test. *p < 0.05, **p < 0.01, ***p < 0.001.
C. Xiang: None; S. Hong: None; B. Zhao: None; H. Pi: None; F. Du: None; X. Lu: None; Y. Tang: None; N. Shen: None; C. Yang: None; R. Wang: None.



