Poster Session C
Rheumatoid arthritis (RA)
Session: (2095–2140) RA – Diagnosis, Manifestations, and Outcomes Poster III
2111: Self-reported Methotrexate Adherence Underestimates Biochemical Adherence: Results from the Methotrexate Use Improvement in Rheumatoid Arthritis Using Biomarker Feedback Trial
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- JB
James Bluett, MBBS
The University of Manchester
Manchester, United KingdomDisclosure information not submitted.
Abstract Poster Presenter(s)
James Bluett1, Kimme Hyrich2, Brian Keevil3, Patty Doran4 and Anne Barton5, 1University of Manchester, Manchester, United Kingdom, 2Versus Arthritis Centre for Epidemiology, Centre for Musculoskeletal Research, The University of Manchester, Manchester, United Kingdom, 3Department of Clinical Biochemistry, Manchester University NHS Foundation Trust, Manchester Academic Health Science Centre, Manchester, United Kingdom, 4School of Social Sciences, The University of Manchester, Manchester, United Kingdom, 5Versus Arthritis Centre for Genetics and Genomics, Centre for Musculoskeletal Research, The University of Manchester, Manchester, United Kingdom
Background/Purpose: Methotrexate (MTX) adherence is suboptimal and is associated with disease flare, DAS-28 response, radiographic damage and healthcare costs. Adherence can be measured using self-reported questionnaires such as the validated Medication Adherence Report Scale (MARS-5). Often the prescriber is blinded to patient non-adherence and self-reported adherence may be an underestimate. Our group has previously developed a sensitive biochemical assay for the detection of MTX adherence, The Methotrexate use Improvement in Rheumatoid Arthritis using Biomarker Feedback (MIRA) is a feasibility trial designed to assess the feasibility of a randomised controlled trial of MTX biochemical adherence biofeedback. Here we investigate the agreement between self-reported and biochemical adherence.
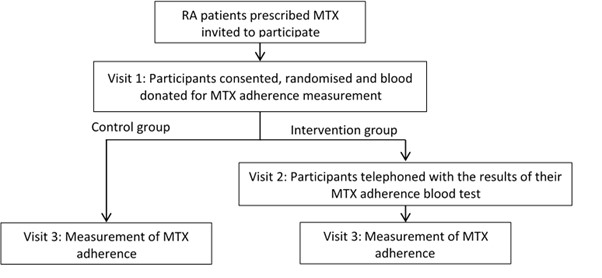
Methods: MIRA is a prospective multi-centre randomised controlled trial investigating the feasibility of a fully powered randomised controlled trial to examine if a biochemical adherence guided intervention is superior to standard clinical care in RA patients. All analyses are exploratory in nature. RA patients prescribed oral MTX for ≥ two years were randomised 1:1 to receive biochemical adherence biofeedback or control (figure 1). Clinico-demographics, biochemical MTX adherence, MARS-5 and DAS-28 were measured at baseline and three months. Self-reported MARS-5 adherence was dichotomised and agreement with biochemical adherence analysed using Cohen's Kappa.
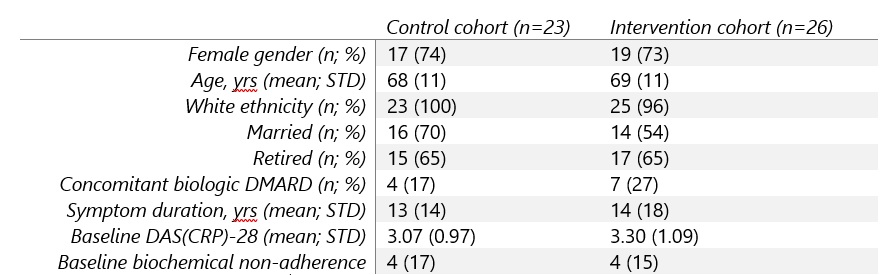
Results: 57 participants were recruited, withdrawal rate was 14% and reasons given were intercurrent illness, lost contact, withdrawn consent and one patient died during follow-up leaving full outcome data available for 49 participants. Baseline clinico-demographics were similar in control and intervention cohorts (Table 1).
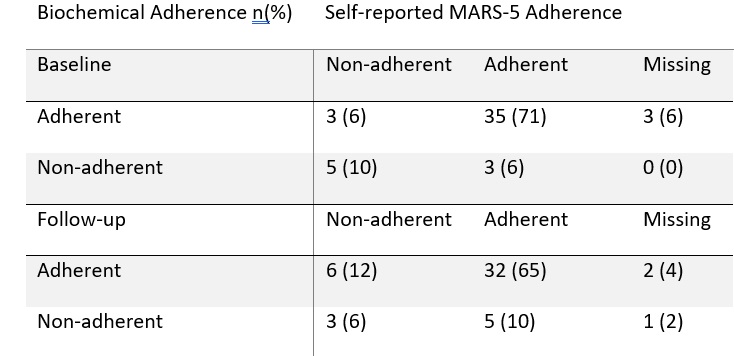
Biochemical adherence worsened in the control cohort and improved in the intervention cohort (83 to 70%, 85 to 92% respectively) whilst change in DAS(CRP)-28 worsened in the control cohort and improved in the intervention cohort (-0.04 STD 1.26 and 0.38 STD 0.78 respectively). Self-reported and biochemical adherence demonstrated fair agreement (κ=0.24) at baseline but poor (κ=0.09) at follow-up (Table 2).
Conclusion: The MIRA trial has shown early evidence of biofeedback improving MTX biochemical adherence and DAS-28 compared to control. Poor agreement between self-reported and biochemical adherence suggests biochemical adherence may be a more clinically useful measure of adherence.
J. Bluett: Fresenius Kabi, 12, Travel/conference fees, Pfizer, 5; K. Hyrich: Abbvie, 6, Bristol-Myers Squibb(BMS), 5, Pfizer, 5; B. Keevil: None; P. Doran: None; A. Barton: Bristol-Myers Squibb(BMS), 5, Pfizer, 5.
Background/Purpose: Methotrexate (MTX) adherence is suboptimal and is associated with disease flare, DAS-28 response, radiographic damage and healthcare costs. Adherence can be measured using self-reported questionnaires such as the validated Medication Adherence Report Scale (MARS-5). Often the prescriber is blinded to patient non-adherence and self-reported adherence may be an underestimate. Our group has previously developed a sensitive biochemical assay for the detection of MTX adherence, The Methotrexate use Improvement in Rheumatoid Arthritis using Biomarker Feedback (MIRA) is a feasibility trial designed to assess the feasibility of a randomised controlled trial of MTX biochemical adherence biofeedback. Here we investigate the agreement between self-reported and biochemical adherence.
Methods: MIRA is a prospective multi-centre randomised controlled trial investigating the feasibility of a fully powered randomised controlled trial to examine if a biochemical adherence guided intervention is superior to standard clinical care in RA patients. All analyses are exploratory in nature. RA patients prescribed oral MTX for ≥ two years were randomised 1:1 to receive biochemical adherence biofeedback or control (figure 1). Clinico-demographics, biochemical MTX adherence, MARS-5 and DAS-28 were measured at baseline and three months. Self-reported MARS-5 adherence was dichotomised and agreement with biochemical adherence analysed using Cohen's Kappa.
Results: 57 participants were recruited, withdrawal rate was 14% and reasons given were intercurrent illness, lost contact, withdrawn consent and one patient died during follow-up leaving full outcome data available for 49 participants. Baseline clinico-demographics were similar in control and intervention cohorts (Table 1).
Biochemical adherence worsened in the control cohort and improved in the intervention cohort (83 to 70%, 85 to 92% respectively) whilst change in DAS(CRP)-28 worsened in the control cohort and improved in the intervention cohort (-0.04 STD 1.26 and 0.38 STD 0.78 respectively). Self-reported and biochemical adherence demonstrated fair agreement (κ=0.24) at baseline but poor (κ=0.09) at follow-up (Table 2).
Conclusion: The MIRA trial has shown early evidence of biofeedback improving MTX biochemical adherence and DAS-28 compared to control. Poor agreement between self-reported and biochemical adherence suggests biochemical adherence may be a more clinically useful measure of adherence.

Figure 1. Trial Flow Chart

Table 1. Baseline clinico-demographics.

Table 2. Biochemical and self-reported adherence at baseline and follow-up
J. Bluett: Fresenius Kabi, 12, Travel/conference fees, Pfizer, 5; K. Hyrich: Abbvie, 6, Bristol-Myers Squibb(BMS), 5, Pfizer, 5; B. Keevil: None; P. Doran: None; A. Barton: Bristol-Myers Squibb(BMS), 5, Pfizer, 5.



