Poster Session C
Systemic lupus erythematosus (SLE)
Session: (2326–2351) SLE – Treatment Poster III
2343: Hydroxychloroquine Discontinuation in the Real World: Reasons, Predictive Factors and Clinical Implications
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- EM
Eduardo Mantovani Cardoso, MD
Beth Israel Deaconess Medical Center
Dorchester, MA, United StatesDisclosure(s): No financial relationships with ineligible companies to disclose
Abstract Poster Presenter(s)
Eduardo Mantovani Cardoso1, Rachel Simon1 and Vasileios Kyttaris2, 1Beth Israel Deaconess Medical Center / Harvard Medical School, Boston, MA, 2BIDMC, Boston, MA
Background/Purpose: Systemic lupus erythematosus (SLE) is a complex autoimmune disease characterized by multi-systemic involvement and difficult-to-predict disease flares. Hydroxychloroquine (HCQ) has long been recognized as a cornerstone of SLE management. However, the impact of discontinuing HCQ on disease activity and flare rates in SLE patients remains a subject of ongoing investigations. We aim to assess factors associated with flares in patients that discontinue HCQ.
Methods: This retrospective cohort study included SLE patients who met the 1997 ACR criteria for SLE. The study period was from 2012 to 2023. Patients were identified from our cohort and were required to have at least one year of follow-up before and after discontinuing HCQ. Thus, the time limit for discontinuation of HCQ was March 2022. Inclusion criteria stipulated consistent HCQ intake without erratic prescription patterns. HCQ discontinuation reason was documented. Patient data were collected on the electronic medical records through chart review. Local IRB approval was granted for it. We evaluated clinical and laboratory parameters historically (profile) and at the index visit, defined as within 3 months of HCQ discontinuation. Flares were evaluated 12 months before and after HCQ discontinuation according to the SLEDAI flare index. Statistical analysis was performed with STATA, group comparison by using t-test for continuous and a chi-square test for categorical variables.
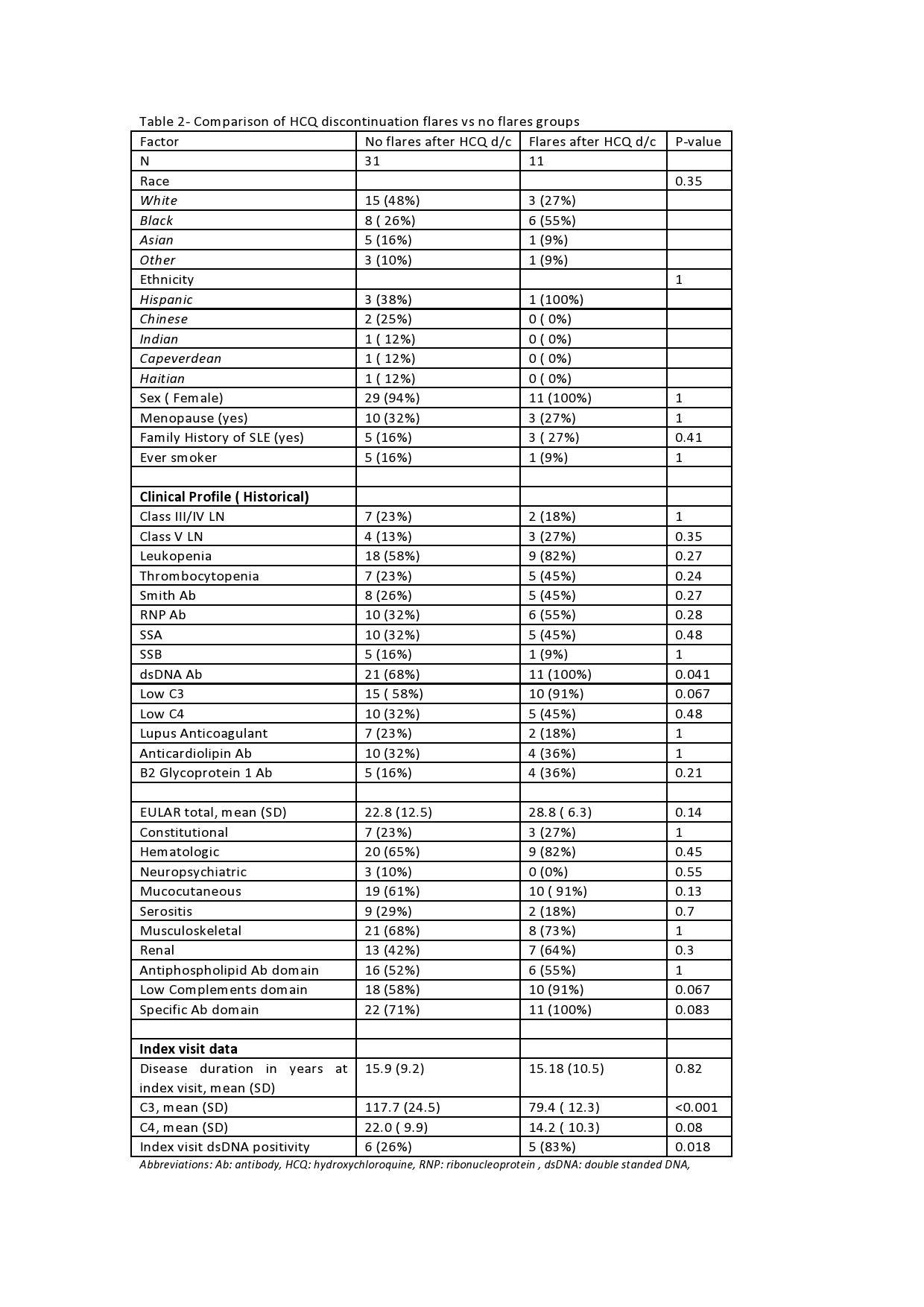
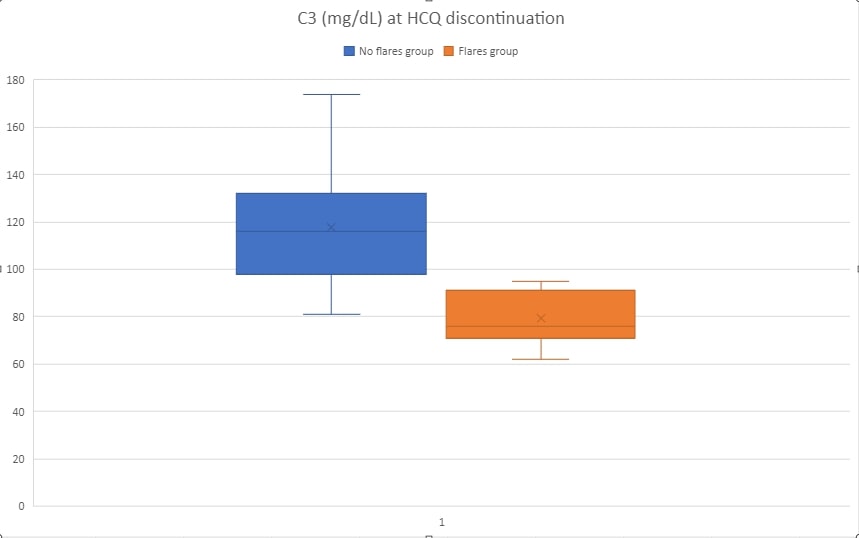
Results: 42 patients were identified that met the established criteria. Ninety-five percent of patients were females. Blacks constituted 33% of the cohort and Hispanics 44%. The mean age at the index visit was 50 years old and were diagnosed, on average, 15 years before the index visit. Common clinical manifestations included mucocutaneous (69%), musculoskeletal (69%), and hematologic (69%). Class III/IV lupus nephritis was present in 21% and Class V in 17% of patients. The immunologic profile included dsDNA Ab positivity (76%), low C3 (67%), RNP antibody (38%), SSA Ab (36%), anticardiolipin Ab (33%), and Smith Ab (31%). ACR/EULAR 2019 classification criteria mean score was 24.4. 19%of patients had flared the year before HCQ discontinuation. The main reasons for discontinuation was retinal toxicity due to HCQ (57%), self-discontinuation (17%), and either other retinopathies or inability to perform eye exams (12%). At the index visit, mean C3 was 108 mg/dL and C4 20 mg/dL, and dsDNA Ab positivity in 38% (Table 1). 11/42 (26.1%) of patients flared the year after HCQ discontinuation, compared to 19% of flares the year before (p-value 0.433). Out of several parameters evaluated for association with flares, the strongest associations were: mean C3 at the index visit ( 79.4 mg/dL in flares versus 117.7 no flares, p-value < 0.001) and dsDNA positivity at index visit ( 83% flares versus 26% no flares, p-value 0.018). Other associations tested are available in Table 2.
Conclusion: In a cohort of 42 SLE patients that had high ACR/EULAR 2019 classification scores ( >20), older age, and prolonged disease duration, flares within 1 year of HCQ discontinuation did not have a higher incidence than the year before HCQ discontinuation but were associated with lower C3 levels and positive dsDNA antibody.

E. Mantovani Cardoso: None; R. Simon: None; V. Kyttaris: AbbVie/Abbott, 5, AstraZeneca, 2, Aurinia, 1, EMD Serono, 5, Exagen, 2, 5, Fresenius Kabi, 1, Horizon Pharmaceuticals, 1, Novartis, 5, Scipher, 1, Takeda, 5, Vertex, 2.
Background/Purpose: Systemic lupus erythematosus (SLE) is a complex autoimmune disease characterized by multi-systemic involvement and difficult-to-predict disease flares. Hydroxychloroquine (HCQ) has long been recognized as a cornerstone of SLE management. However, the impact of discontinuing HCQ on disease activity and flare rates in SLE patients remains a subject of ongoing investigations. We aim to assess factors associated with flares in patients that discontinue HCQ.
Methods: This retrospective cohort study included SLE patients who met the 1997 ACR criteria for SLE. The study period was from 2012 to 2023. Patients were identified from our cohort and were required to have at least one year of follow-up before and after discontinuing HCQ. Thus, the time limit for discontinuation of HCQ was March 2022. Inclusion criteria stipulated consistent HCQ intake without erratic prescription patterns. HCQ discontinuation reason was documented. Patient data were collected on the electronic medical records through chart review. Local IRB approval was granted for it. We evaluated clinical and laboratory parameters historically (profile) and at the index visit, defined as within 3 months of HCQ discontinuation. Flares were evaluated 12 months before and after HCQ discontinuation according to the SLEDAI flare index. Statistical analysis was performed with STATA, group comparison by using t-test for continuous and a chi-square test for categorical variables.
Results: 42 patients were identified that met the established criteria. Ninety-five percent of patients were females. Blacks constituted 33% of the cohort and Hispanics 44%. The mean age at the index visit was 50 years old and were diagnosed, on average, 15 years before the index visit. Common clinical manifestations included mucocutaneous (69%), musculoskeletal (69%), and hematologic (69%). Class III/IV lupus nephritis was present in 21% and Class V in 17% of patients. The immunologic profile included dsDNA Ab positivity (76%), low C3 (67%), RNP antibody (38%), SSA Ab (36%), anticardiolipin Ab (33%), and Smith Ab (31%). ACR/EULAR 2019 classification criteria mean score was 24.4. 19%of patients had flared the year before HCQ discontinuation. The main reasons for discontinuation was retinal toxicity due to HCQ (57%), self-discontinuation (17%), and either other retinopathies or inability to perform eye exams (12%). At the index visit, mean C3 was 108 mg/dL and C4 20 mg/dL, and dsDNA Ab positivity in 38% (Table 1). 11/42 (26.1%) of patients flared the year after HCQ discontinuation, compared to 19% of flares the year before (p-value 0.433). Out of several parameters evaluated for association with flares, the strongest associations were: mean C3 at the index visit ( 79.4 mg/dL in flares versus 117.7 no flares, p-value < 0.001) and dsDNA positivity at index visit ( 83% flares versus 26% no flares, p-value 0.018). Other associations tested are available in Table 2.
Conclusion: In a cohort of 42 SLE patients that had high ACR/EULAR 2019 classification scores ( >20), older age, and prolonged disease duration, flares within 1 year of HCQ discontinuation did not have a higher incidence than the year before HCQ discontinuation but were associated with lower C3 levels and positive dsDNA antibody.
Table 1- Table 1- Clinical and serological profile of the SLE patients.
Abbreviations: Ab: antibody, HCQ: hydroxychloroquine, LFT: liver function tests, RNP: ribonucleoprotein , dsDNA: double standed DNA,
Abbreviations: Ab: antibody, HCQ: hydroxychloroquine, LFT: liver function tests, RNP: ribonucleoprotein , dsDNA: double standed DNA,

Table 2- Comparison of HCQ discontinuation flares vs no flares groups

C3 levels and SLE flares after HCQ discontinuation
E. Mantovani Cardoso: None; R. Simon: None; V. Kyttaris: AbbVie/Abbott, 5, AstraZeneca, 2, Aurinia, 1, EMD Serono, 5, Exagen, 2, 5, Fresenius Kabi, 1, Horizon Pharmaceuticals, 1, Novartis, 5, Scipher, 1, Takeda, 5, Vertex, 2.



