Poster Session C
Epidemiology, health policy and outcomes
Session: (1895–1912) Measures & Measurement of Healthcare Quality Poster II
1910: Development of the American College of Rheumatology Toolkit for Implementation of Rheumatoid Arthritis Outcome Measures in Clinical Practice
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- CN
Catherine Nasrallah, BS, MPH (she/her/hers)
University of California San Francisco (UCSF)
San Francisco, CA, United StatesDisclosure information not submitted.
Abstract Poster Presenter(s)
Catherine Nasrallah1, Lindsay Jacobsohn1, Gabriela Schmajuk2, Emma Kersey1, Cammie Young3, Cammie Young3, Janell Martin4, Lori Barber5, Jennifer Barton6, Puneet Bajaj7, Christie M. Bartels8, Sancia Ferguson9, Elizabeth Wahl10, Kimberly DeQuattro11, Patti Katz12, Maria I. (\"Maio\") Danila13, Meera Subash14, Christina Downey15, JoAnn Zell16, Kimberly Reiter17, Elena Weinstein18 and Jinoos Yazdany1, 1University of California San Francisco, San Francisco, CA, 2UCSF / SFVA, San Francisco, CA, 3University of California San Francisco, Oakland, CA, 4American College of Rheumatology, Atlanta, GA, 5ACR, Atlanta, GA, 6VA Portland Health Care System/OHSU, Portland, OR, 7UT Southwestern Medical Center, Dallas, TX, 8University of Wisconsin, School of Medicine and Public Health, Madison, WI, 9University of Wisconsin, Madison, WI, 10VA Puget Sound Healthcare System, Seattle, WA, 11University of Pennsylvania, Media, PA, 12University of California San Francisco, San Rafael, CA, 13University of Alabama at Birmingham (UAB), Birmingham VA Medical Center, Birmingham, AL, 14University of Texas Health Science Center at Houston, Houston, TX, 15Loma Linda University Medical Center, Loma Linda, CA, 16University of Colorado Anschutz Medical Campus, Denver, CO, 17Albuquerque VA Medical Center, Albuquerque, NM, 18University of Colorado, Englewood, CO
Background/Purpose: Despite significant interest in the scale and spread of rheumatoid arthritis (RA) outcome measures to facilitate a patient-centered, treat-to-target approach, use of these measures remains inconsistent among rheumatologists. We used the Consolidated Framework for Implementation Research (CFIR) to guide qualitative interviews with stakeholders about facilitators and barriers to RA outcome measurement in real-world rheumatology care, with the goal of disseminating best practices for implementation via an online toolkit
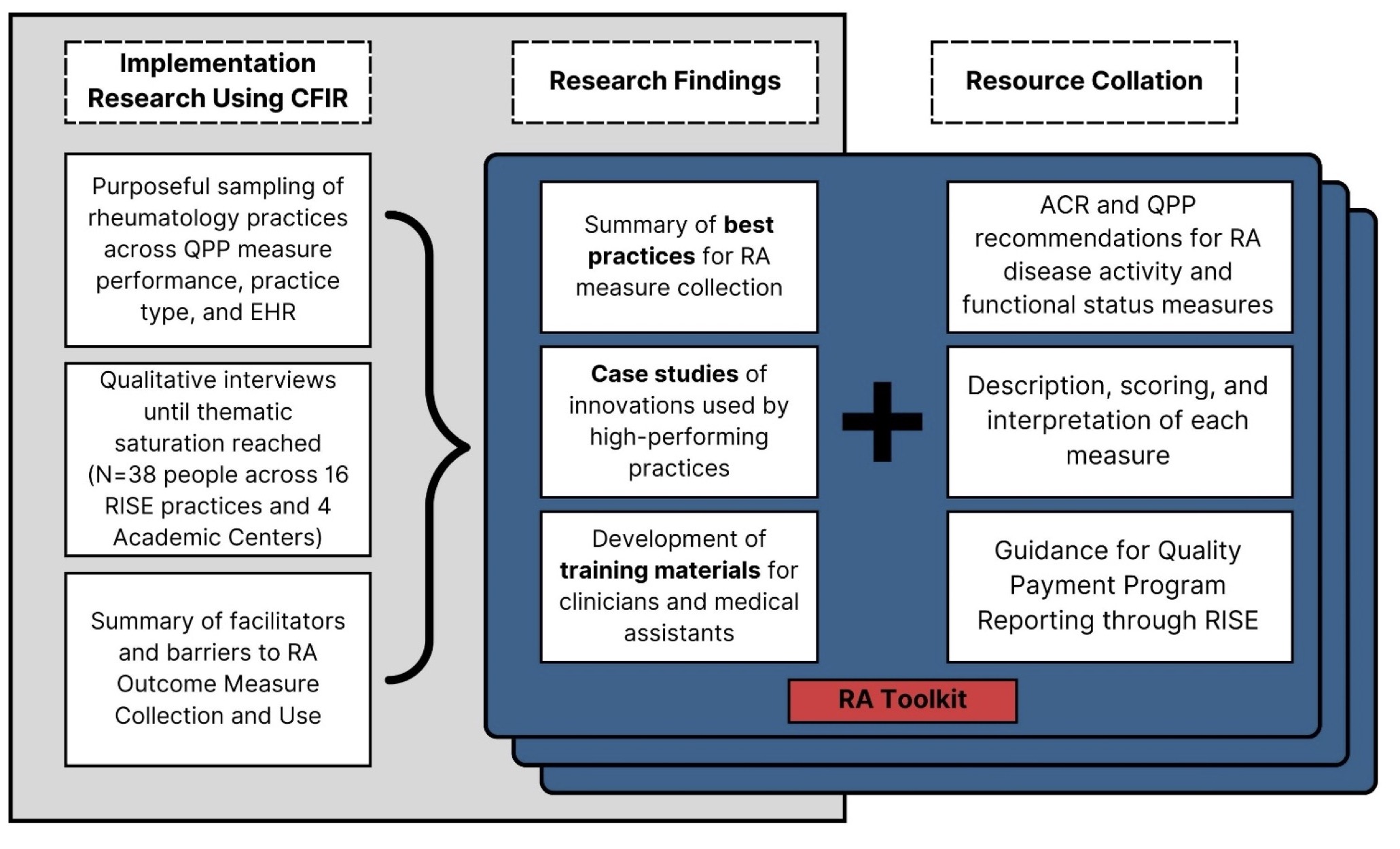
Methods: Using RISE registry data, we invited 85 practices with high performance on RA disease activity (DA) measures (≥ 50% percentile on the Quality Payment Program (QPP) 177 periodic assessment of DA) and 54 practices with lower performance to participate. Because academic medical centers (AMCs) are under-represented in RISE, 4 AMCs were also invited to participate. Rheumatologists and key staff from each practice participated in virtual interviews. CFIR concepts were used to develop a semi-structured interview guide to understand workflows, facilitators, and barriers to RA outcome measure collection. Interview recordings were transcribed verbatim and analyzed thematically using deductive and inductive techniques to generate themes and subthemes. Collaborating with the ACR staff, we compiled information from these interviews to develop the online RA Toolkit, a comprehensive resource to facilitate RA outcome measurement in clinical practice (Figure 1).
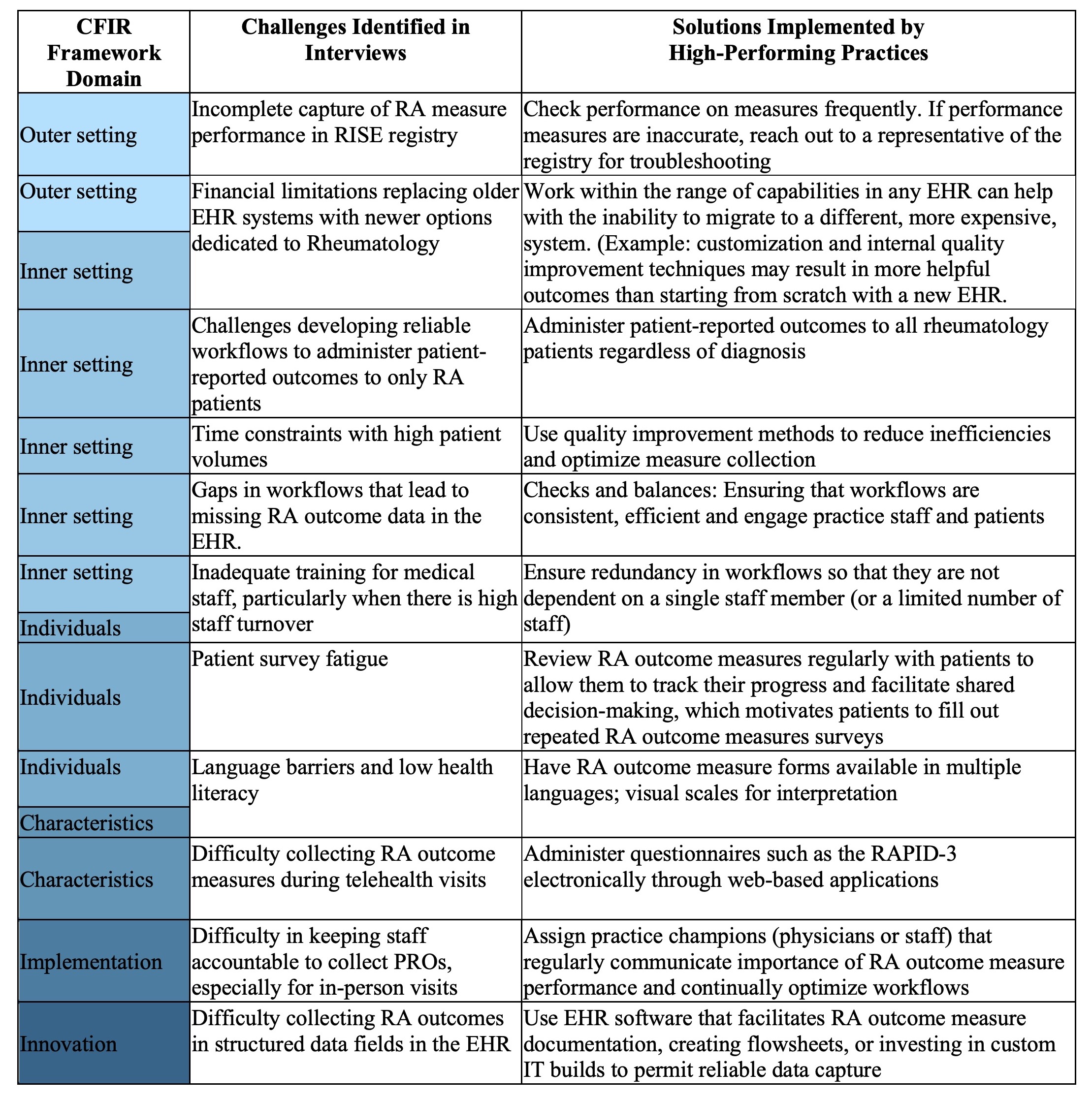
Results: Interviews were conducted with invited practices until thematic saturation was reached (38 interviews across 16 RISE practices and 4 AMC). Participants included 21 rheumatologists, 8 medical assistants, 6 practice managers, 1 nurse practitioner, and 2 information technology specialists. Each interview highlighted unique innovations, resources, best practices, and challenges for collecting RA outcome measures (select findings summarized in Table 1). Results of the qualitative interviews, ACR guidelines regarding RA outcome measures, and other evidence-based resources were compiled in the RA Toolkit (available at RAToolkit.kotobee.com). The Toolkit is a multimedia online resource for RA outcome measure implementation geared toward use by clinicians and staff. Sections include text, videos, and downloadable forms that aid in understanding and using RA outcome measures in clinical practice, recommendations and best practices identified from high performers across diverse practice settings and EHRs, training resources for staff, and validated translations of available RA measures in Spanish and Chinese (Figure 2).
Conclusion: Qualitative interviews conducted with rheumatology teams laid the groundwork to develop a best practices implementation toolkit to disseminate innovations in RA measures collection. The ACR RA Toolkit is the first comprehensive, evidence-based, open-source resource available for rheumatologists and healthcare teams to guide effective implementation, collection, and use of RA outcome measures that promote quality and value-based patient-centered care.

C. Nasrallah: None; L. Jacobsohn: None; G. Schmajuk: None; E. Kersey: None; C. Young: None; C. Young: None; J. Martin: None; L. Barber: None; J. Barton: None; P. Bajaj: None; C. Bartels: Pfizer, 5; S. Ferguson: None; E. Wahl: None; K. DeQuattro: None; P. Katz: None; M. Danila: Horizon, 5, Pfizer, 5, RheumNow, 2, UCB, 2; M. Subash: None; C. Downey: None; J. Zell: None; K. Reiter: None; E. Weinstein: Priovant, 5; J. Yazdany: Astra Zeneca, 2, 5, Aurinia, 5, Gilead, 5, Pfizer, 2.
Background/Purpose: Despite significant interest in the scale and spread of rheumatoid arthritis (RA) outcome measures to facilitate a patient-centered, treat-to-target approach, use of these measures remains inconsistent among rheumatologists. We used the Consolidated Framework for Implementation Research (CFIR) to guide qualitative interviews with stakeholders about facilitators and barriers to RA outcome measurement in real-world rheumatology care, with the goal of disseminating best practices for implementation via an online toolkit
Methods: Using RISE registry data, we invited 85 practices with high performance on RA disease activity (DA) measures (≥ 50% percentile on the Quality Payment Program (QPP) 177 periodic assessment of DA) and 54 practices with lower performance to participate. Because academic medical centers (AMCs) are under-represented in RISE, 4 AMCs were also invited to participate. Rheumatologists and key staff from each practice participated in virtual interviews. CFIR concepts were used to develop a semi-structured interview guide to understand workflows, facilitators, and barriers to RA outcome measure collection. Interview recordings were transcribed verbatim and analyzed thematically using deductive and inductive techniques to generate themes and subthemes. Collaborating with the ACR staff, we compiled information from these interviews to develop the online RA Toolkit, a comprehensive resource to facilitate RA outcome measurement in clinical practice (Figure 1).
Results: Interviews were conducted with invited practices until thematic saturation was reached (38 interviews across 16 RISE practices and 4 AMC). Participants included 21 rheumatologists, 8 medical assistants, 6 practice managers, 1 nurse practitioner, and 2 information technology specialists. Each interview highlighted unique innovations, resources, best practices, and challenges for collecting RA outcome measures (select findings summarized in Table 1). Results of the qualitative interviews, ACR guidelines regarding RA outcome measures, and other evidence-based resources were compiled in the RA Toolkit (available at RAToolkit.kotobee.com). The Toolkit is a multimedia online resource for RA outcome measure implementation geared toward use by clinicians and staff. Sections include text, videos, and downloadable forms that aid in understanding and using RA outcome measures in clinical practice, recommendations and best practices identified from high performers across diverse practice settings and EHRs, training resources for staff, and validated translations of available RA measures in Spanish and Chinese (Figure 2).
Conclusion: Qualitative interviews conducted with rheumatology teams laid the groundwork to develop a best practices implementation toolkit to disseminate innovations in RA measures collection. The ACR RA Toolkit is the first comprehensive, evidence-based, open-source resource available for rheumatologists and healthcare teams to guide effective implementation, collection, and use of RA outcome measures that promote quality and value-based patient-centered care.

Figure 1: Methods for Developing the RA Outcome Measures Toolkit

Table 1: Summary of key findings from qualitative interviews about RA outcome measure collection with rheumatology practices

Figure 2: RA Measures toolkit: https://ratoolkit.kotobee.com/
C. Nasrallah: None; L. Jacobsohn: None; G. Schmajuk: None; E. Kersey: None; C. Young: None; C. Young: None; J. Martin: None; L. Barber: None; J. Barton: None; P. Bajaj: None; C. Bartels: Pfizer, 5; S. Ferguson: None; E. Wahl: None; K. DeQuattro: None; P. Katz: None; M. Danila: Horizon, 5, Pfizer, 5, RheumNow, 2, UCB, 2; M. Subash: None; C. Downey: None; J. Zell: None; K. Reiter: None; E. Weinstein: Priovant, 5; J. Yazdany: Astra Zeneca, 2, 5, Aurinia, 5, Gilead, 5, Pfizer, 2.



