Poster Session C
Rheumatoid arthritis (RA)
Session: (2095–2140) RA – Diagnosis, Manifestations, and Outcomes Poster III
2110: Rheumatoid Arthritis and Changes in Pulmonary Function Measures on Spirometry in a Prospective Longitudinal Cohort of Smokers
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- KH
Keigo Hayashi, MD, PhD, MPH
Brigham and Women's Hospital
Brookline, MA, United StatesDisclosure information not submitted.
Abstract Poster Presenter(s)
Keigo Hayashi1, Gregory McDermott1, Pierre-Antoine Juge2, Matthew Moll1, Michael Cho1, Tracy J. Doyle1, Gregory Kinney3, Danielle Sansone-Poe3, Kendra Young3, Paul Dellaripa4, Zachary Wallace5, Elizabeth Regan6, Gary Hunninghake1, Edwin Silverman1, Samuel Ash1, Raul San Jose Estepar1, George Washko1 and Jeffrey Sparks7, 1Brigham and Women's Hospital, Boston, MA, 2Division of Rheumatology, Inflammation, and Immunity Brigham & Women's Hospital, Boston, MA, 3University of Colorado Denver, Denver, CO, 4Brigham and Women's Hospital, Boston, MA, 5Massachusetts General Hospital, Newton, MA, 6National Jewish Health, Denver, CO, 7Division of Rheumatology, Inflammation, and Immunity, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA
Background/Purpose: Rheumatoid arthritis (RA) has known extra-articular manifestations that can result in restrictive and obstructive patterns on pulmonary function measures, especially in smokers. However, investigations comparing RA and non-RA for pulmonary function change have not been performed. In this study, we compared longitudinal spirometry measures between smokers with and without RA.
Methods: We analyzed longitudinal data from COPDGene, a multicenter prospective cohort of smokers with at least 10 pack-years. We investigated longitudinal pulmonary function measures in people with RA compared to non-RA comparators and healthy non-smokers. Spirometry was conducted at baseline (2007-2011) and at a second visit 5 years later. RA cases were identified by self-reported RA and DMARD use (PPV 88%); non-RA comparators reported no RA and no DMARD use. The outcomes were the annual change in postbronchodilator percent predicted forced expiratory volume in one second (%FEV1), percent predicted forced vital capacity (%FVC), and FEV1/FVC ratio. We compared these outcomes between RA cases and non-RA comparators using linear regression, adjusted for age, sex, BMI, smoking status (current/past), pack-years, baseline spirometry results, and inhaled/systemic medication use for obstructive lung diseases. An additional analysis used inverse probability of censoring weighting (IPCW) to account for possible differential censoring, defined by drop-out or death before the follow-up visit was due. We also stratified the analysis by the presence of obstructive pattern (FEV1/FVC< 0.7) at baseline.
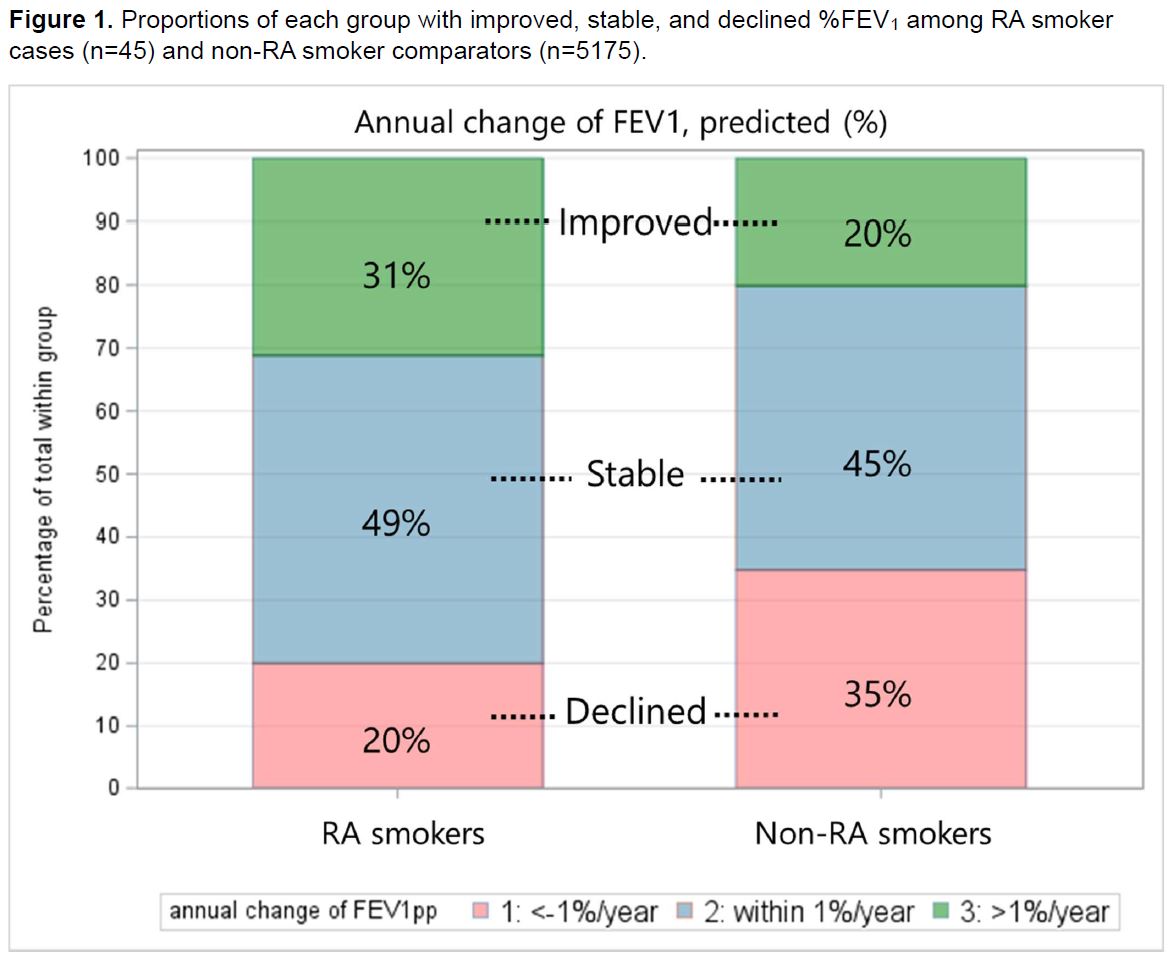
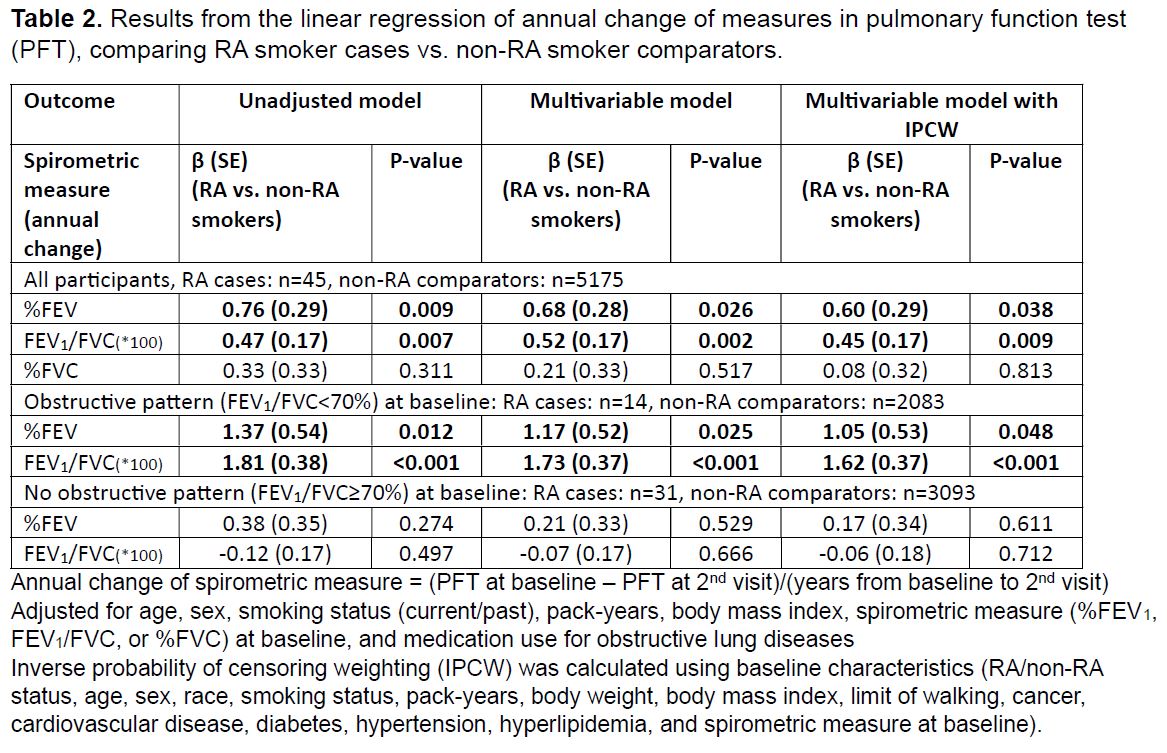
Results: We analyzed 45 RA smoker cases and 5175 non-RA smoker comparators with available follow-up spirometry data (Table 1). The mean %FEV1 at baseline was 77% in RA cases and 80% in non-RA comparators. The mean change in %FEV1 from baseline to the 5-year follow-up was +1.8% in RA cases and -2.3% in non-RA comparators. Proportions of each group with improved, stable, and declined %FEV1 are shown in Figure 1. In the multivariable linear regression models, both %FEV1 and FEV1/FVC showed significantly less decline in RA cases compared to non-RA comparators (%FEV1: β 0.68, p=0.026; FEV1/FVC: β 0.52, p=0.002; Table 2). This result was more prominent in participants with obstructive pattern at baseline (RA vs. non-RA: %FEV1: β 1.17, p=0.0025; FEV1/FVC: β 1.73, p< 0.001). There was no significant difference in the annual change in %FVC between the two groups. Results were similar after accounting for possible differential loss to follow-up or death.
Conclusion: In this first comparative study among smokers to examine longitudinal pulmonary function in RA, RA cases were less likely to have %FEV1 and FEV1/FVC declines than non-RA comparators. Results were strongest among RA cases with baseline obstructive defect and not explained by differences in smoking, suggesting that RA with obstruction may be a unique phenotype. Further studies are needed to replicate with larger sample size and to examine mechanisms and potential for reversibility related to systemic inflammation, autoimmunity, and RA treatment.
.jpg)
K. Hayashi: None; G. McDermott: None; P. Juge: None; M. Moll: Bayer, 5; M. Cho: Bayer, 5, GlaxoSmithKlein(GSK), 5; T. Doyle: None; G. Kinney: None; D. Sansone-Poe: None; K. Young: None; P. Dellaripa: None; Z. Wallace: BioCryst, 2, Bristol-Myers Squibb(BMS), 5, Horizon, 1, 2, 5, MedPace, 2, Novartis, 1, PPD, 2, Sanofi, 1, 5, Shionogi, 1, Visterra, 1, 2, Zenas, 1, 2; E. Regan: None; G. Hunninghake: Boehringer-Ingelheim, 2, Chugai Pharamaceuticals, 2, Gerson Lehrman Group, 2; E. Silverman: Bayer, 5; S. Ash: Boehringer-Ingelheim, 5, Quantitative Imaging Solutions, 8, 10, Verona Pharmaceuticals, 1; R. San Jose Estepar: Boehringer-Ingelheim, 5, Chiesi, 6, Imbio, 9, Insmed, 5, Leuko Labs, 2, Lung Biotechnology, 5; G. Washko: Boehringer-Ingelheim, 5, Janssen, 2, Novartis, 2, Pulmonx, 2, Quantitative Imaging Solutions, 8, Vertex, 2; J. Sparks: AbbVie, 2, Amgen, 2, Boehringer Ingelheim, 2, Bristol-Myers Squibb, 2, 5, Gilead, 2, Inova Diagnostics, 2, Janssen, 2, Optum, 2, Pfizer, 2, ReCor, 2.
Background/Purpose: Rheumatoid arthritis (RA) has known extra-articular manifestations that can result in restrictive and obstructive patterns on pulmonary function measures, especially in smokers. However, investigations comparing RA and non-RA for pulmonary function change have not been performed. In this study, we compared longitudinal spirometry measures between smokers with and without RA.
Methods: We analyzed longitudinal data from COPDGene, a multicenter prospective cohort of smokers with at least 10 pack-years. We investigated longitudinal pulmonary function measures in people with RA compared to non-RA comparators and healthy non-smokers. Spirometry was conducted at baseline (2007-2011) and at a second visit 5 years later. RA cases were identified by self-reported RA and DMARD use (PPV 88%); non-RA comparators reported no RA and no DMARD use. The outcomes were the annual change in postbronchodilator percent predicted forced expiratory volume in one second (%FEV1), percent predicted forced vital capacity (%FVC), and FEV1/FVC ratio. We compared these outcomes between RA cases and non-RA comparators using linear regression, adjusted for age, sex, BMI, smoking status (current/past), pack-years, baseline spirometry results, and inhaled/systemic medication use for obstructive lung diseases. An additional analysis used inverse probability of censoring weighting (IPCW) to account for possible differential censoring, defined by drop-out or death before the follow-up visit was due. We also stratified the analysis by the presence of obstructive pattern (FEV1/FVC< 0.7) at baseline.
Results: We analyzed 45 RA smoker cases and 5175 non-RA smoker comparators with available follow-up spirometry data (Table 1). The mean %FEV1 at baseline was 77% in RA cases and 80% in non-RA comparators. The mean change in %FEV1 from baseline to the 5-year follow-up was +1.8% in RA cases and -2.3% in non-RA comparators. Proportions of each group with improved, stable, and declined %FEV1 are shown in Figure 1. In the multivariable linear regression models, both %FEV1 and FEV1/FVC showed significantly less decline in RA cases compared to non-RA comparators (%FEV1: β 0.68, p=0.026; FEV1/FVC: β 0.52, p=0.002; Table 2). This result was more prominent in participants with obstructive pattern at baseline (RA vs. non-RA: %FEV1: β 1.17, p=0.0025; FEV1/FVC: β 1.73, p< 0.001). There was no significant difference in the annual change in %FVC between the two groups. Results were similar after accounting for possible differential loss to follow-up or death.
Conclusion: In this first comparative study among smokers to examine longitudinal pulmonary function in RA, RA cases were less likely to have %FEV1 and FEV1/FVC declines than non-RA comparators. Results were strongest among RA cases with baseline obstructive defect and not explained by differences in smoking, suggesting that RA with obstruction may be a unique phenotype. Further studies are needed to replicate with larger sample size and to examine mechanisms and potential for reversibility related to systemic inflammation, autoimmunity, and RA treatment.
.jpg)
Table 1. Baseline characteristics of RA cases, non-RA comparators and healthy non-smokers (n=5220)

Figure 1. Proportions of each group with improved, stable, and declined %FEV1 among RA smoker cases (n=45) and non-RA smoker comparators (n=5175).

Table 2. Results from the linear regression of annual change of measures in pulmonary function test
(PFT), comparing RA smoker cases vs. non-RA smoker comparators.
(PFT), comparing RA smoker cases vs. non-RA smoker comparators.
K. Hayashi: None; G. McDermott: None; P. Juge: None; M. Moll: Bayer, 5; M. Cho: Bayer, 5, GlaxoSmithKlein(GSK), 5; T. Doyle: None; G. Kinney: None; D. Sansone-Poe: None; K. Young: None; P. Dellaripa: None; Z. Wallace: BioCryst, 2, Bristol-Myers Squibb(BMS), 5, Horizon, 1, 2, 5, MedPace, 2, Novartis, 1, PPD, 2, Sanofi, 1, 5, Shionogi, 1, Visterra, 1, 2, Zenas, 1, 2; E. Regan: None; G. Hunninghake: Boehringer-Ingelheim, 2, Chugai Pharamaceuticals, 2, Gerson Lehrman Group, 2; E. Silverman: Bayer, 5; S. Ash: Boehringer-Ingelheim, 5, Quantitative Imaging Solutions, 8, 10, Verona Pharmaceuticals, 1; R. San Jose Estepar: Boehringer-Ingelheim, 5, Chiesi, 6, Imbio, 9, Insmed, 5, Leuko Labs, 2, Lung Biotechnology, 5; G. Washko: Boehringer-Ingelheim, 5, Janssen, 2, Novartis, 2, Pulmonx, 2, Quantitative Imaging Solutions, 8, Vertex, 2; J. Sparks: AbbVie, 2, Amgen, 2, Boehringer Ingelheim, 2, Bristol-Myers Squibb, 2, 5, Gilead, 2, Inova Diagnostics, 2, Janssen, 2, Optum, 2, Pfizer, 2, ReCor, 2.



