Poster Session C
Rheumatoid arthritis (RA)
Session: (2095–2140) RA – Diagnosis, Manifestations, and Outcomes Poster III
2108: Specific Symptom Clusters at Diagnosis Signal a Poorer Early RA Prognosis: Data from the Canadian Early Arthritis Cohort (CATCH)
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- SB
Abstract Poster Presenter(s)
Susan Bartlett1, Clifton Bingham2, Orit Schieir3, Marie-France Valois4, Janet Pope5, Louis Bessette6, Gilles Boire7, Carol Hitchon8, Edward Keystone9, Carter Thorne10, Diane Tin11, Glen Hazlewood12 and Vivian Bykerk13, 1McGill University, Montreal, QC, Canada, 2Johns Hopkins Medicine, Baltimore, MD, 3McGill University, Montréal, QC, Canada, 4McGill University, Pointe-Claire, QC, Canada, 5University of Western Ontario, London, ON, Canada, 6Centre de l'Ostéoporose et de Rhumatologie de Québec, Quebec City, QC, Canada, 7Université de Sherbrooke, Sherbrooke, QC, Canada, 8University of Manitoba, Winnipeg, MB, Canada, 9Keystone Consulting Enterprises Inc., Toronto, ON, Canada, 10Southlake Regional Health Centre, Newmarket, ON, Canada, 11Newmarket Rheumatology Consultants, Newmarket, ON, Canada, 12University of Calgary, Calgary, AB, Canada, 13Hospital for Special Surgery, New York, NY
Background/Purpose: Symptom clusters are stable groups of 2+ symptoms that are related to each other and frequently co-occur. Identifying symptom clusters in early RA may point to underlying mechanisms and RA subtypes, help predict disease trajectories and personalize symptom management to improve outcomes. We used scores from PROMIS-29 to identify symptom clusters at diagnosis in MTX-naïve patients starting MTX therapy. We evaluated the stability of these clusters and likelihood of transitioning between clusters over the first 6 months of treatment.
Methods: Data were from new onset RA patients (sx < 1 year) enrolled in the Canadian Early Arthritis Cohort (CATCH) who started MTX and had clinical and PROM measures available at 0, 3, and 6-months. Based on empirical and qualitative data, we used latent class analyses to identify symptom clusters from PROMIS-29 physical (pain, fatigue, sleep) and emotional (depression, anxiety) scales (levels: minimal, mild, moderate, severe). Models were compared using AIC, BIC, G-square and log-likelihoods. We estimated transitioning between clusters at 3- and 6-months using latent transition analyses.
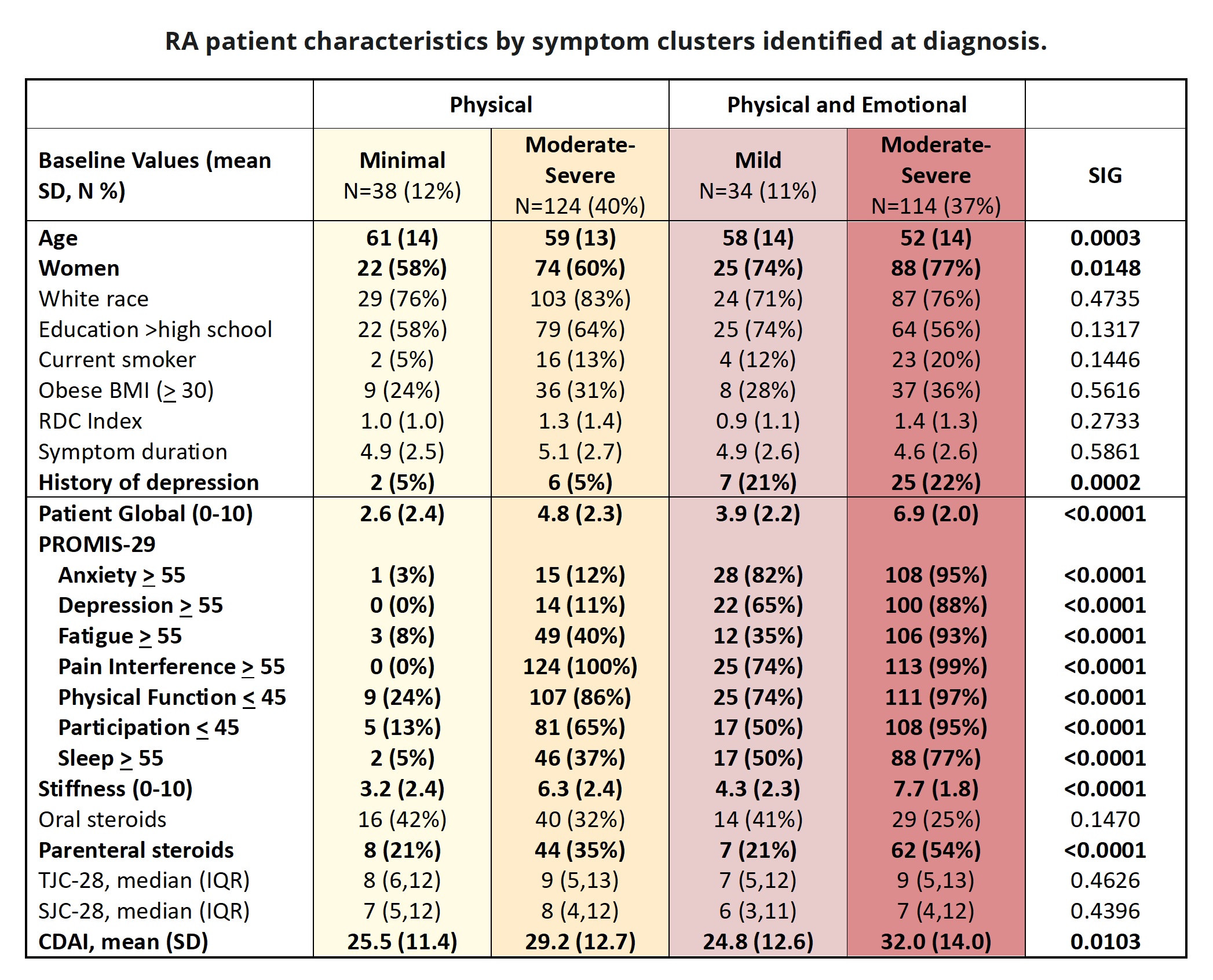
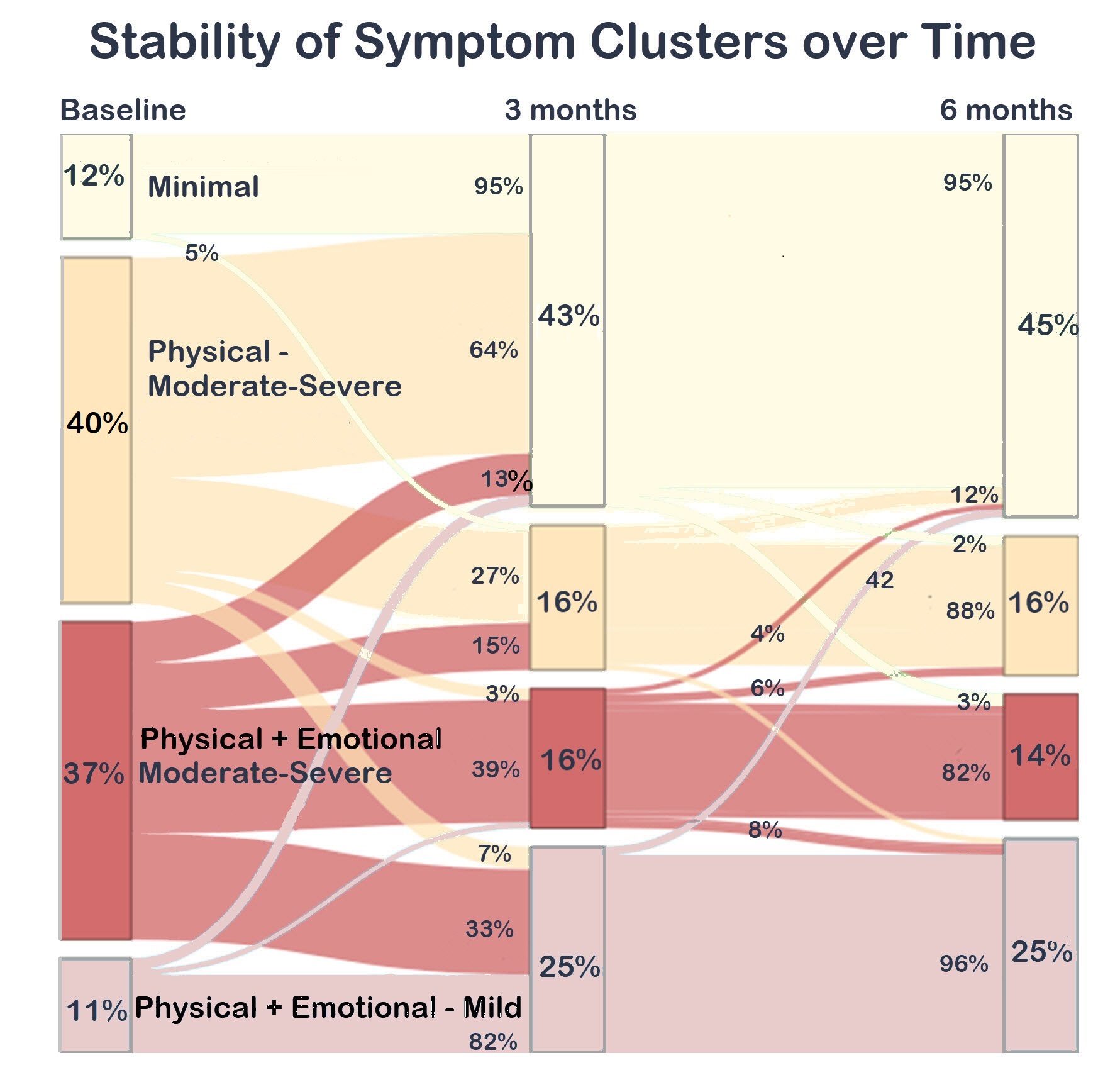
Results: Of 310 adults with new RA, followed for 6 months and starting MTX, 67% were women and 78%White. Participants had a mean age of 56 yrs, CDAI 29.3, and RA symptom durationof 5 months. Optimal clusters included pain, fatigue, anxiety and depression and three intensity levels. We identified 4 discrete symptom clusters at diagnosis:MINIMAL (12%); Moderate-Severe Physical (M-S P: 40%); Mild Physical + Emotional (MILD P+E: 11%); Moderate-Severe Physical + Emotional (M-S P+E 37%). Clusters had similar sociodemographics except the MINIMALclass were slightly older and fewer were women; M-S P+E had a greater likelihood of depression history (Table). SJC and TJC were similar among classes; worse symptoms were associated with higher CDAI. Classes with emotional sx had significantly worse mood and sleep, and the M-S P class were more likely to report a history of depression. More patients with moderate-severe symptoms were on parenteral steroids. Most transitions occurred over the first 3 months. The best prognosis was for the MINIMAL class; 95% were classified similarly at 3 and 6 months. Among those with MILD P+E , almost all were classified similarly at 3 (82%) and 6 (96%) months. Participants with M-S P at diagnosis were also likely to transition to MINIMAL (64%) or MILD P+E (7%). The worse prognosis was among those with M-S P+E; at 3 months, a minority transitioned to MINIMAL (13%) or MILD P+E (33%) classes. Patients with either mild or moderate-severe emotional symptoms were the least likely to transition to milder symptom classes.
Conclusion: We identified 4 ERA patient subsets with varying physical (pain, fatigue) and emotional (depression, anxiety) symptoms and evaluated transitions over 6-months of initial MTX treatment. At baseline, most participants had moderate-severe Physical +/- Emotional symptoms. Patient subsets with emotional symptoms at RA-diagnosis were less likely to transition to better controlled symptom classes. Our results suggest that co-occurring emotional symptoms at diagnosis may reflect important subsets and signal a more guarded RA prognosis over the first 6 months.
S. Bartlett: Janssen, 6, Merck/MSD, 2, 6, Novartis, 2, Organon, 1, 6, PROMIS Health Organization, 4, Sandoz, 2, 6; C. Bingham: None; O. Schieir: None; M. Valois: None; J. Pope: AbbVie, 1, 2; L. Bessette: AbbVie, 2, 5, 6, Amgen, 2, 5, 6, Bristol Myers Squibb, 2, 5, 6, Eli Lilly, 2, 5, 6, Fresenius Kabi, 2, 6, Gilead, 2, 5, 6, JAMP Pharma, 2, 5, 6, Janssen, 2, 5, 6, Novartis, 2, 5, 6, Organon, 2, 6, Pfizer, 2, 5, 6, Sandoz, 2, 6, Sanofi, 2, 5, 6, Teva, 2, 6, UCB, 2, 5, 6, UCBA, 5; G. Boire: Eli Lilly, 1, Janssen, 6, Organon, 1, Orimed Pharma, 1, 6, Otsuka, 1, Pfizer, 1, 5, Sandoz, 1, Teva, 1, Viatris, 1, 6; C. Hitchon: Astra Zeneca, 1, Pfizer, 5; E. Keystone: AbbVie/Abbott, 2, 6, Amgen, 2, 6, celltrion, 2, 6, Eli Lilly, 2, 6, Fresenius Kabi, 2, 6, Pfizer, 2, 6, Samsung Bioepsis, 2, sandoz, 2, 6; C. Thorne: Abbvie, 1, Biogen, 2, Nordic Pharma, 1, Pfizer, 1, 5, Roche, 1, Sandoz, 1, 2; D. Tin: None; G. Hazlewood: None; V. Bykerk: Abbvie, 2, BMS, 2, Pfizer, 2.
Background/Purpose: Symptom clusters are stable groups of 2+ symptoms that are related to each other and frequently co-occur. Identifying symptom clusters in early RA may point to underlying mechanisms and RA subtypes, help predict disease trajectories and personalize symptom management to improve outcomes. We used scores from PROMIS-29 to identify symptom clusters at diagnosis in MTX-naïve patients starting MTX therapy. We evaluated the stability of these clusters and likelihood of transitioning between clusters over the first 6 months of treatment.
Methods: Data were from new onset RA patients (sx < 1 year) enrolled in the Canadian Early Arthritis Cohort (CATCH) who started MTX and had clinical and PROM measures available at 0, 3, and 6-months. Based on empirical and qualitative data, we used latent class analyses to identify symptom clusters from PROMIS-29 physical (pain, fatigue, sleep) and emotional (depression, anxiety) scales (levels: minimal, mild, moderate, severe). Models were compared using AIC, BIC, G-square and log-likelihoods. We estimated transitioning between clusters at 3- and 6-months using latent transition analyses.
Results: Of 310 adults with new RA, followed for 6 months and starting MTX, 67% were women and 78%White. Participants had a mean age of 56 yrs, CDAI 29.3, and RA symptom durationof 5 months. Optimal clusters included pain, fatigue, anxiety and depression and three intensity levels. We identified 4 discrete symptom clusters at diagnosis:MINIMAL (12%); Moderate-Severe Physical (M-S P: 40%); Mild Physical + Emotional (MILD P+E: 11%); Moderate-Severe Physical + Emotional (M-S P+E 37%). Clusters had similar sociodemographics except the MINIMALclass were slightly older and fewer were women; M-S P+E had a greater likelihood of depression history (Table). SJC and TJC were similar among classes; worse symptoms were associated with higher CDAI. Classes with emotional sx had significantly worse mood and sleep, and the M-S P class were more likely to report a history of depression. More patients with moderate-severe symptoms were on parenteral steroids. Most transitions occurred over the first 3 months. The best prognosis was for the MINIMAL class; 95% were classified similarly at 3 and 6 months. Among those with MILD P+E , almost all were classified similarly at 3 (82%) and 6 (96%) months. Participants with M-S P at diagnosis were also likely to transition to MINIMAL (64%) or MILD P+E (7%). The worse prognosis was among those with M-S P+E; at 3 months, a minority transitioned to MINIMAL (13%) or MILD P+E (33%) classes. Patients with either mild or moderate-severe emotional symptoms were the least likely to transition to milder symptom classes.
Conclusion: We identified 4 ERA patient subsets with varying physical (pain, fatigue) and emotional (depression, anxiety) symptoms and evaluated transitions over 6-months of initial MTX treatment. At baseline, most participants had moderate-severe Physical +/- Emotional symptoms. Patient subsets with emotional symptoms at RA-diagnosis were less likely to transition to better controlled symptom classes. Our results suggest that co-occurring emotional symptoms at diagnosis may reflect important subsets and signal a more guarded RA prognosis over the first 6 months.


S. Bartlett: Janssen, 6, Merck/MSD, 2, 6, Novartis, 2, Organon, 1, 6, PROMIS Health Organization, 4, Sandoz, 2, 6; C. Bingham: None; O. Schieir: None; M. Valois: None; J. Pope: AbbVie, 1, 2; L. Bessette: AbbVie, 2, 5, 6, Amgen, 2, 5, 6, Bristol Myers Squibb, 2, 5, 6, Eli Lilly, 2, 5, 6, Fresenius Kabi, 2, 6, Gilead, 2, 5, 6, JAMP Pharma, 2, 5, 6, Janssen, 2, 5, 6, Novartis, 2, 5, 6, Organon, 2, 6, Pfizer, 2, 5, 6, Sandoz, 2, 6, Sanofi, 2, 5, 6, Teva, 2, 6, UCB, 2, 5, 6, UCBA, 5; G. Boire: Eli Lilly, 1, Janssen, 6, Organon, 1, Orimed Pharma, 1, 6, Otsuka, 1, Pfizer, 1, 5, Sandoz, 1, Teva, 1, Viatris, 1, 6; C. Hitchon: Astra Zeneca, 1, Pfizer, 5; E. Keystone: AbbVie/Abbott, 2, 6, Amgen, 2, 6, celltrion, 2, 6, Eli Lilly, 2, 6, Fresenius Kabi, 2, 6, Pfizer, 2, 6, Samsung Bioepsis, 2, sandoz, 2, 6; C. Thorne: Abbvie, 1, Biogen, 2, Nordic Pharma, 1, Pfizer, 1, 5, Roche, 1, Sandoz, 1, 2; D. Tin: None; G. Hazlewood: None; V. Bykerk: Abbvie, 2, BMS, 2, Pfizer, 2.



