Poster Session B
Periodic fever syndromes, autoinflammatory diseases, Still’s disease and MAS/HLH
Session: (1124–1154) Miscellaneous Rheumatic & Inflammatory Diseases Poster II
1128: Refractory Inflammatory Ocular Pathology and Treatment with Janus Kinase Inhibitors. Multicenter Study and Literature Review
Monday, November 13, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- CL
Carmen Lasa Teja, MD
Hospital Universitario Marqués de Valdecilla
Anero, SpainDisclosure information not submitted.
Abstract Poster Presenter(s)
Carmen Lasa-Teja1, Lara Sanchez-Bilbao2, Jose Luis Martin-Varillas3, Vanesa Calvo Río4, José Luis Álvarez-Vega5, Emma Beltran-Catalan6, María del Mar Esteban-Ortega7, Santiago Muñoz7, Olga Maiz8, Ignacio Torre-Salaberri9, Ana Urruticoechea10, Elia Valls-Pascual11, Raúl Veroz12, Carmen Alvarez Reguera13, Rosalía Demetrio13 and Ricardo Blanco14, 1Hospital Universitario Marqués de Valdecilla. IDIVAL, Immunopathology Group, Santander, Spain, 2Hospital Universitario Marques de Valdecilla, Santander, Spain, 3Hospital de Laredo, Laredo, Spain, 4Valdecilla Hospital, Santander, Spain, 5Complejo Hospitalario Universitario de Badajoz, Badajoz, Spain, 6HOSPITAL DEL MAR, Barcelona, Spain, 7Hospital Infanta Sofia, Madrid, Spain, 8Hospital Universitario de Donostia. San Sebastián, Spain., Donosti, Spain, 9Hospital Universitario de Basurto, Bilbao, Spain, 10Hospital Can Misses, Ibiza, Spain, 11Hospital Universitario Doctor Peset. Alicante, Spain., Valencia, Spain, 12Hospital de Mérida, Mérida. Spain., Mérida, Spain, 13Hospital Universitario Marqués de Valdecilla, Santander, Spain, 14Hospital Universitario Marqués de Valdecilla, IDIVAL, Santander, Spain
Background/Purpose: Inflammatory ocular pathology (IOP) includes internal (uveitis) and external [mainly ocular surface pathology such as epi/scleritis and peripheral ulcerative keratitis (PUK)] involvement. IOP may be severe ocular conditions refractory to conventional immunosuppressants and even biological therapy. Janus Kinase inhibitors (JAKINIB) had shown efficacy in refractory cases of different immune-mediated inflammatory diseases (IMID).
In patients with refractory IOP treated with JAKINIB our aims were a) to assess the patients of Spanish referral centers, b) Literature review.
Methods: Multicenter study of 11 patients with refractory IOP treated with JAKINIB. For Literature review a search was conducted in PubMed, Embase and the Cochrane library from their inception to 1st January 2023. Original research articles studying JAKINIB treatment in patients with IOP were included. In addition, a therapeutical approach of refractory IOP is proposed.
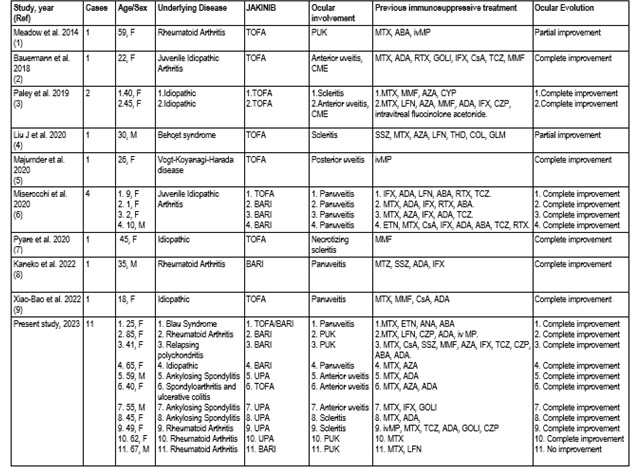
Results: We have identified 11 cases in eight University Hospitals and 13 cases in the literature review. These 24 patients (17 women/ 7 men) (35 affected eyes), mean age 38.9±21.9 years, had different refractory IOP (uveitis=14; scleritis=5, PUK=5). Most of IOP were associated with IMID (n=19, 79.2%) while 5 cases (20.8%.), were idiopathic. The main underlying IMID were rheumatoid arthritis (n=6, 25%), juvenile idiopathic arthritis (n=5, 20.8%) and spondyloarthritis (n=4; 16.7%) (TABLE). Uveitis (n=14) followed by ocular surface pathology (n=10) were the most frequent subtypes of IOP. Patterns of uveitis were panuveitis (n=8), anterior uveitis (n=5; 2 of them with Cystoid macular), and posterior (n=1). Ocular surface pathology was due to scleritis (n=5) and PUK (n=5). In addition to systemic corticosteroids, before JAKINIB, conventional (n= 23; 95.8%) and biologicalimmunosuppressive drugs (n=18; 75%) were required. The JAKINIB most widely used was tofacitinib (n= 11; 45.8%) followed by baricitinib (n=8; 33.3%). In one patient with Blau Syndrome and uveitis, tofacitinib was switched to baricitinib due to severe lymphopenia.In two other patients, UPA was discontinued due to anemia and skin adverse reaction. Finally, only in one patient baricitinib was withdrawn due to lack of improvement. After starting JAKINIB treatment, 23 patients presented clinical improvement, complete (n=21, 87.5%) or partial (n= 2; 8.3%). Based on these data a therapeutical approach of refractory IOP was proposed (FIGURE).
Conclusion: JAKINIB may be an effective and safe therapy in IOP refractory to conventional or even biological immunosuppressive therapy.
.jpg)
C. Lasa-Teja: None; L. Sanchez-Bilbao: None; J. Martin-Varillas: None; V. Calvo Río: None; J. Álvarez-Vega: None; E. Beltran-Catalan: None; M. Esteban-Ortega: None; S. Muñoz: None; O. Maiz: None; I. Torre-Salaberri: None; A. Urruticoechea: None; E. Valls-Pascual: None; R. Veroz: None; C. Alvarez Reguera: None; R. Demetrio: None; R. Blanco: AbbVie, 5, 6, Amgen, 6, AstraZeneca, 2, BMS, 6, Eli Lilly, 6, Galapagos, 2, 6, Janssen, 2, 6, MSD, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche, 5, 6, Sanofi, 6.
Background/Purpose: Inflammatory ocular pathology (IOP) includes internal (uveitis) and external [mainly ocular surface pathology such as epi/scleritis and peripheral ulcerative keratitis (PUK)] involvement. IOP may be severe ocular conditions refractory to conventional immunosuppressants and even biological therapy. Janus Kinase inhibitors (JAKINIB) had shown efficacy in refractory cases of different immune-mediated inflammatory diseases (IMID).
In patients with refractory IOP treated with JAKINIB our aims were a) to assess the patients of Spanish referral centers, b) Literature review.
Methods: Multicenter study of 11 patients with refractory IOP treated with JAKINIB. For Literature review a search was conducted in PubMed, Embase and the Cochrane library from their inception to 1st January 2023. Original research articles studying JAKINIB treatment in patients with IOP were included. In addition, a therapeutical approach of refractory IOP is proposed.
Results: We have identified 11 cases in eight University Hospitals and 13 cases in the literature review. These 24 patients (17 women/ 7 men) (35 affected eyes), mean age 38.9±21.9 years, had different refractory IOP (uveitis=14; scleritis=5, PUK=5). Most of IOP were associated with IMID (n=19, 79.2%) while 5 cases (20.8%.), were idiopathic. The main underlying IMID were rheumatoid arthritis (n=6, 25%), juvenile idiopathic arthritis (n=5, 20.8%) and spondyloarthritis (n=4; 16.7%) (TABLE). Uveitis (n=14) followed by ocular surface pathology (n=10) were the most frequent subtypes of IOP. Patterns of uveitis were panuveitis (n=8), anterior uveitis (n=5; 2 of them with Cystoid macular), and posterior (n=1). Ocular surface pathology was due to scleritis (n=5) and PUK (n=5). In addition to systemic corticosteroids, before JAKINIB, conventional (n= 23; 95.8%) and biologicalimmunosuppressive drugs (n=18; 75%) were required. The JAKINIB most widely used was tofacitinib (n= 11; 45.8%) followed by baricitinib (n=8; 33.3%). In one patient with Blau Syndrome and uveitis, tofacitinib was switched to baricitinib due to severe lymphopenia.In two other patients, UPA was discontinued due to anemia and skin adverse reaction. Finally, only in one patient baricitinib was withdrawn due to lack of improvement. After starting JAKINIB treatment, 23 patients presented clinical improvement, complete (n=21, 87.5%) or partial (n= 2; 8.3%). Based on these data a therapeutical approach of refractory IOP was proposed (FIGURE).
Conclusion: JAKINIB may be an effective and safe therapy in IOP refractory to conventional or even biological immunosuppressive therapy.

.jpg)
FIGURE. Therapeutical approach of refractory inflammatory ocular pathology.
C. Lasa-Teja: None; L. Sanchez-Bilbao: None; J. Martin-Varillas: None; V. Calvo Río: None; J. Álvarez-Vega: None; E. Beltran-Catalan: None; M. Esteban-Ortega: None; S. Muñoz: None; O. Maiz: None; I. Torre-Salaberri: None; A. Urruticoechea: None; E. Valls-Pascual: None; R. Veroz: None; C. Alvarez Reguera: None; R. Demetrio: None; R. Blanco: AbbVie, 5, 6, Amgen, 6, AstraZeneca, 2, BMS, 6, Eli Lilly, 6, Galapagos, 2, 6, Janssen, 2, 6, MSD, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche, 5, 6, Sanofi, 6.



