Poster Session C
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (2227–2256) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III: SpA
2238: Longitudinal Effect of Guselkumab on Biomarkers of Inflammation and Cardiovascular Risk in Bionaive Patients with Active Psoriatic Arthritis and High Systemic Inflammatory Burden: Post-hoc Analysis of a Phase 3, Randomized, Double-blind, Placebo-Controlled Study
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
Abstract Poster Presenter(s)
Arthur Kavanaugh1, Enrique Soriano2, Jan Dutz3, Carlo Selmi4, Emmanouil Rampakakis5, Natalie shiff6, Francois Nantel7, Frederic Lavie8 and Laura Coates9, 1Division of Rheumatology, Allergy, and Immunology, University of California San Diego, La Jolla, CA, 2Rheumatology Section, Internal Medicine Services, Hospital Italiano de Buenos Aires, and University Institute Hospital Italiano de Buenos Aires, Buenos Aires, Argentina, 3Department of Dermatology and Skin Science, Vancouver General Hospital, Vancouver, BC, Canada, 4Rheumatology and Clinical Immunology, Humanitas Research Hospital / Internal Medicine, Humanitas University, Rozzano, Italy, 5McGill University, Department of Pediatrics / JSS Medical Research, Scientific Affairs, Montreal, QC, Canada, 6Immunology, Janssen Scientific Affairs, LLC / Adjunct, Community Health and Epidemiology, University of Saskatchewan, Horsham, PA, 7Nantel Medsci Consult, Consultant, Montreal, QC, Canada, 8The Janssen Pharmaceutical Companies of Johnson & Johnson, Paris, France, 9University of Oxford, Oxford, United Kingdom
Background/Purpose: Psoriatic arthritis (PsA) has been associated with an increased risk of cardiovascular (CV) disease, likely due to accelerated atherosclerosis secondary to chronic inflammation.1 As such, patients (pts) and their health care providers have high interest in modification of CV risk. Neutrophil-to-lymphocyte ratio (NLR) and high-sensitivity (hs) CRP are biomarkers of systemic inflammation that can be followed over time and may serve as markers of CV risk. Guselkumab (GUS), a fully human IL-23p19-subunit inhibitor, has demonstrated efficacy in treating multiple PsA domains and a favorable safety profile.2,3 This analysis assessed the impact of GUS on NLR and hsCRP as markers of CV risk in subsets of pts with active PsA and high systemic inflammatory burden.
Methods: DISCOVER-2 enrolled bionaïve adults with active PsA (≥5 swollen and ≥5 tender joints, and hsCRP ≥0.6 mg/dL) despite standard therapies; pts were randomized 1:1:1 to GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at W0, W4, Q8W; or placebo (PBO) with crossover to GUS 100 mg Q4W at W24 (PBO àGUS). The cohorts of DISCOVER-2 pts included in this analysis had baseline (BL) NLR values >2.5 (High NLR)4 or BL hsCRP levels >10 mg/L (High hsCRP).5 Changes from BL through W24 in NLR and hsCRP were assessed with mixed models for repeated measures adjusting for BL values of NLR and hsCRP, respectively, and BL use of conventional synthetic DMARDs, corticosteroids, and NSAIDs. Systolic (SBP) and diastolic (DBP) blood pressure as well as body mass index (BMI) through W100 were assessed descriptively.
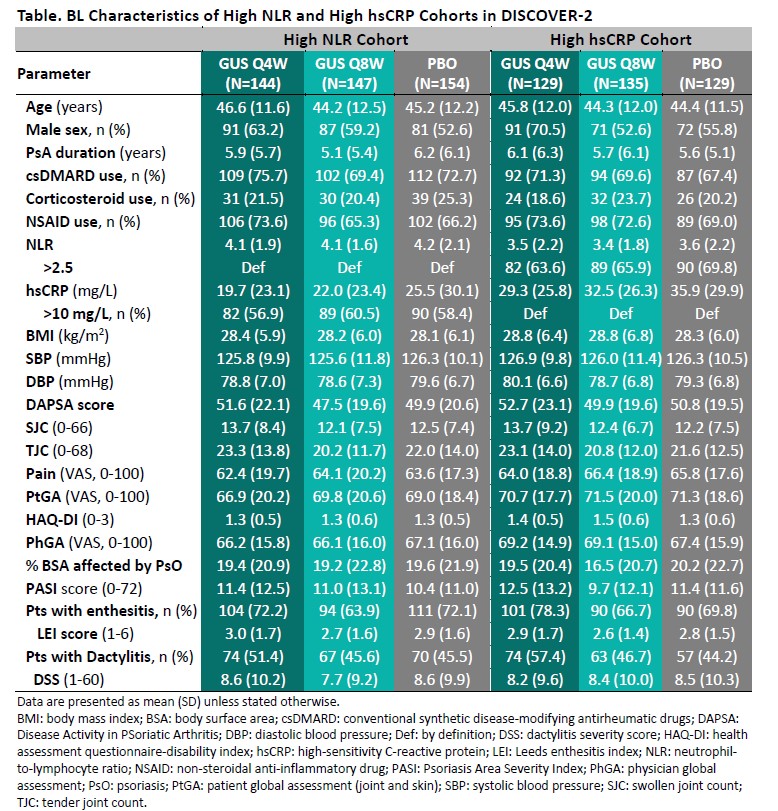
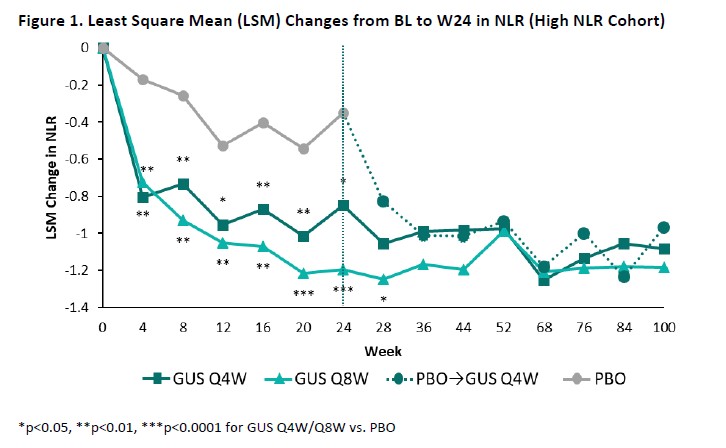
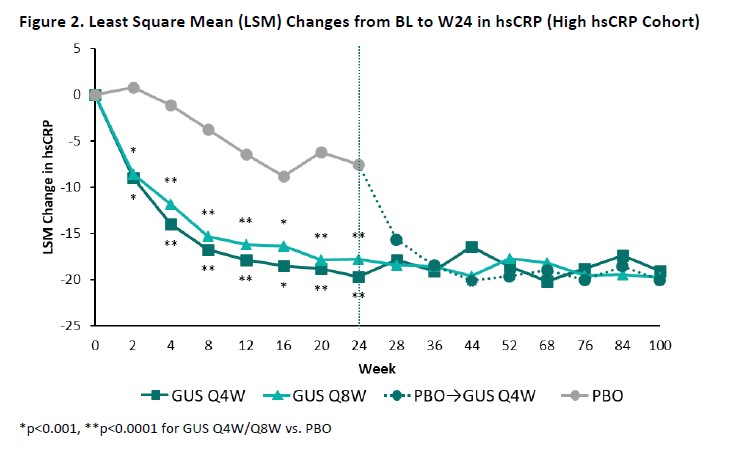
Results: The High NLR and High hsCRP cohorts comprised 445 and 393 patients, respectively, with approximately 60% of pts in each cohort also meeting the criteria for the other; BL characteristics (other than NLR/hsCRP) were generally consistent both between cohorts and across randomized treatment groups (Table). Upon adjusting for BL values and concomitant PsA medications, GUS-treated pts exhibited significantly greater reductions compared with PBO in both NLR (Figure 1) and hsCRP (Figure 2) as of the first timepoint assessed (W4) and through W24, with PBOà GUS quickly (W28) converging following crossover to GUS. Reduced NLR and hsCRP levels (p< 0.0001 vs BL) were sustained through 2 years of GUS treatment. In both cohorts, mean BMI, SBP, and DBP remained stable through up to 2 years of GUS treatment, irrespective of GUS dosing regimen (data not shown).
Conclusion: In these two subgroups of bionaïve PsA pts with high levels of biomarkers of inflammation and CV risk, GUS led to rapid and sustained reductions in NLR and hs-CRP. In addition, traditional CV risk factors remained stable for 2 years. These findings highlight the potential utility of GUS to ameliorate systemic inflammation associated with elevated CV risk and support a consistent guselkumab safety profile in patients with PsA and high systemic inflammatory burden.
References
1. Zheng Z. Front Cardiovasc Med. 2022 Apr 6;9:83543
2. Deodhar A. Lancet. 2020 Apr 4;395:1115
3. Mease PJ. Lancet. 2020 Apr 4;395:1126
4. Qiao S. Ther Clin Risk Manag. 2020; 16: 437
5. Merola JF. Rheumatol Ther. 2022 Jun;9:935
A. Kavanaugh: Amgen, 2, BMS, 2, Eli Lilly, 2, Novartis, 2, Pfizer, 2, UCB, 2; E. Soriano: AbbVie, 2, 5, 6, Amgen, 6, Bristol-Myers Squibb, 6, Eli Lilly, 6, Janssen, 2, 5, 6, Novartis, 2, 5, 6, Pfizer, 5, 6, Roche, 2, 5, 6, UCB, 5, 6; J. Dutz: AbbVie, 6, Amgen, 6, Bausch, 6, Boehringer-Ingelheim, 1, Bristol-Myers Squibb(BMS), 6, Corbus, 5, Eli Lilly, 5, Janssen, 6, Leo, 6, Novartis, 6, Pfizer, 6, Sanofi, 6, Solius, 5; C. Selmi: AbbVie, 2, 5, 6, Alfa-Wassermann, 2, 6, Amgen, 2, 5, 6, Biogen, 2, 6, Eli Lilly, 2, 6, Galapagos, 2, 6, Janssen, 2, 6, Novartis, 2, 6, Pfizer, 5, SOBI, 2, 6; E. Rampakakis: Janssen, 2, JSS Medical Research, Inc, 3; N. shiff: AbbVie, 11, Gilead, 11, Iovance, 11, Janssen Scientific Affairs, LLC, 3, Johnson & Johnson, 11, Novo-Nordisc, 11, Pfizer, 11; F. Nantel: Janssen, 2, Johnson & Johnson, 11; F. Lavie: Janssen, 3, Johnson & Johnson, 11; L. Coates: AbbVie, 2, 5, 6, Amgen, 2, 5, 6, Biogen, 6, Bristol Myers Squibb, 2, Celgene, 2, 5, 6, Eli Lilly, 2, 5, 6, Galapagos, 2, 6, Gilead Sciences, 2, 6, GSK, 6, Janssen, 2, 5, 6, Medac, 6, MoonLake, 2, Novartis, 2, 5, 6, Pfizer Inc, 2, 5, 6, UCB, 2, 5, 6.
Background/Purpose: Psoriatic arthritis (PsA) has been associated with an increased risk of cardiovascular (CV) disease, likely due to accelerated atherosclerosis secondary to chronic inflammation.1 As such, patients (pts) and their health care providers have high interest in modification of CV risk. Neutrophil-to-lymphocyte ratio (NLR) and high-sensitivity (hs) CRP are biomarkers of systemic inflammation that can be followed over time and may serve as markers of CV risk. Guselkumab (GUS), a fully human IL-23p19-subunit inhibitor, has demonstrated efficacy in treating multiple PsA domains and a favorable safety profile.2,3 This analysis assessed the impact of GUS on NLR and hsCRP as markers of CV risk in subsets of pts with active PsA and high systemic inflammatory burden.
Methods: DISCOVER-2 enrolled bionaïve adults with active PsA (≥5 swollen and ≥5 tender joints, and hsCRP ≥0.6 mg/dL) despite standard therapies; pts were randomized 1:1:1 to GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at W0, W4, Q8W; or placebo (PBO) with crossover to GUS 100 mg Q4W at W24 (PBO àGUS). The cohorts of DISCOVER-2 pts included in this analysis had baseline (BL) NLR values >2.5 (High NLR)4 or BL hsCRP levels >10 mg/L (High hsCRP).5 Changes from BL through W24 in NLR and hsCRP were assessed with mixed models for repeated measures adjusting for BL values of NLR and hsCRP, respectively, and BL use of conventional synthetic DMARDs, corticosteroids, and NSAIDs. Systolic (SBP) and diastolic (DBP) blood pressure as well as body mass index (BMI) through W100 were assessed descriptively.
Results: The High NLR and High hsCRP cohorts comprised 445 and 393 patients, respectively, with approximately 60% of pts in each cohort also meeting the criteria for the other; BL characteristics (other than NLR/hsCRP) were generally consistent both between cohorts and across randomized treatment groups (Table). Upon adjusting for BL values and concomitant PsA medications, GUS-treated pts exhibited significantly greater reductions compared with PBO in both NLR (Figure 1) and hsCRP (Figure 2) as of the first timepoint assessed (W4) and through W24, with PBOà GUS quickly (W28) converging following crossover to GUS. Reduced NLR and hsCRP levels (p< 0.0001 vs BL) were sustained through 2 years of GUS treatment. In both cohorts, mean BMI, SBP, and DBP remained stable through up to 2 years of GUS treatment, irrespective of GUS dosing regimen (data not shown).
Conclusion: In these two subgroups of bionaïve PsA pts with high levels of biomarkers of inflammation and CV risk, GUS led to rapid and sustained reductions in NLR and hs-CRP. In addition, traditional CV risk factors remained stable for 2 years. These findings highlight the potential utility of GUS to ameliorate systemic inflammation associated with elevated CV risk and support a consistent guselkumab safety profile in patients with PsA and high systemic inflammatory burden.
References
1. Zheng Z. Front Cardiovasc Med. 2022 Apr 6;9:83543
2. Deodhar A. Lancet. 2020 Apr 4;395:1115
3. Mease PJ. Lancet. 2020 Apr 4;395:1126
4. Qiao S. Ther Clin Risk Manag. 2020; 16: 437
5. Merola JF. Rheumatol Ther. 2022 Jun;9:935



A. Kavanaugh: Amgen, 2, BMS, 2, Eli Lilly, 2, Novartis, 2, Pfizer, 2, UCB, 2; E. Soriano: AbbVie, 2, 5, 6, Amgen, 6, Bristol-Myers Squibb, 6, Eli Lilly, 6, Janssen, 2, 5, 6, Novartis, 2, 5, 6, Pfizer, 5, 6, Roche, 2, 5, 6, UCB, 5, 6; J. Dutz: AbbVie, 6, Amgen, 6, Bausch, 6, Boehringer-Ingelheim, 1, Bristol-Myers Squibb(BMS), 6, Corbus, 5, Eli Lilly, 5, Janssen, 6, Leo, 6, Novartis, 6, Pfizer, 6, Sanofi, 6, Solius, 5; C. Selmi: AbbVie, 2, 5, 6, Alfa-Wassermann, 2, 6, Amgen, 2, 5, 6, Biogen, 2, 6, Eli Lilly, 2, 6, Galapagos, 2, 6, Janssen, 2, 6, Novartis, 2, 6, Pfizer, 5, SOBI, 2, 6; E. Rampakakis: Janssen, 2, JSS Medical Research, Inc, 3; N. shiff: AbbVie, 11, Gilead, 11, Iovance, 11, Janssen Scientific Affairs, LLC, 3, Johnson & Johnson, 11, Novo-Nordisc, 11, Pfizer, 11; F. Nantel: Janssen, 2, Johnson & Johnson, 11; F. Lavie: Janssen, 3, Johnson & Johnson, 11; L. Coates: AbbVie, 2, 5, 6, Amgen, 2, 5, 6, Biogen, 6, Bristol Myers Squibb, 2, Celgene, 2, 5, 6, Eli Lilly, 2, 5, 6, Galapagos, 2, 6, Gilead Sciences, 2, 6, GSK, 6, Janssen, 2, 5, 6, Medac, 6, MoonLake, 2, Novartis, 2, 5, 6, Pfizer Inc, 2, 5, 6, UCB, 2, 5, 6.




