Poster Session C
Systemic lupus erythematosus (SLE)
Session: (2257–2325) SLE – Diagnosis, Manifestations, & Outcomes Poster III
2264: Proposal for Defining Moderate and Severe Activity States in Systemic Lupus Erythematosus. Impact on Flares and Other Outcomes
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- IA
Irene Altabas Gonzalez, MD
Complejo Hospitalario de Vigo
Vigo, SpainDisclosure information not submitted.
Abstract Poster Presenter(s)
Ivonne Lourdes Mamani Velarde1, Iñigo Rúa-Figueroa2, Sara García Pérez1, Irene Altabás González3, CORAL Mourino Rodriguez4, Norman Jiménez5, JULIA MARTINEZ BARRIO6, Maria Galindo-Izquierdo7, Jaime Calvo- Alén8, Celia Eurasquin9, Belen Serrano Benavente10, Esther Uriarte Isacelaya11, Eva Tomero Muriel12, Mercedes Freire González13, Ricardo Blanco14, Eva Salgado-Pérez15, Paloma Vela16, Antonio Fernandez-Nebro17, Alejandro Olivé-Marqués18, Clara Sanguesa Gomez19, Javier Narvaez20, Raúl Menor-Almagro21, Jose Rosas22, José Ángel Hernández Beriain23, JAVIER MANERO24, Elena Aurrecoechea Aguinaga25, Oihane Ibarguengoitia-Barrena26, Carlos Montilla-Morales27, Gema Bonilla28, Vicente Torrente-Segarra29, Ana Paula Cacheda30, Maria J. García-Villanueva31, Clara Moriano Morales32, Concepción Fito Manteca33, Nuria Lozano Rivas34, Cristina Bohórquez35 and Jose-Maria Pego-Reigosa36, 1University Hospital of Vigo, Department of Rheumatology. IRIDIS Group (Investigation in Rheumatology and Immune-Diseases), Galicia Sur Health Research Institute (IISGS), Vigo, Spain, 2Rheumatology, Hospital de Gran Canaria Doctor Negrin, Las Palmas de Gran Canaria, Spain, 3Department of Rheumatology, University Hospital of Vigo. IRIDIS Group (Investigation in Rheumatology and Immune-Diseases), Vigo, Spain, 4Complejo Hospitalario Universitario de Vigo. IRIDIS (Investigation in Rheumatology and Immune-Mediated Diseases) Group, Galicia Sur Health Research Institute, Vigo, Spain, 5IIRIDIS (Investigation in Rheumatology and Immune-Mediated Diseases) Group, Galicia Sur Health Research Institute., Vigo, Spain, 6Rheumatology, Gregorio Marañon University Hospital, Madrid, Spain, 7Rheumatology, University Hospital of 12 de Octubre, Madrid, Spain, 8Rheumatology, Bioaraba Research Unit, Hospital Universitario Araba, Vitoria, Spain, 9Department of Rheumatology, Doctor Negrín University Hospital, Las Palmas de Gran Canaria, Spain, 10Rheumatology Department Hospital Gregorio Marañón, Madrid, Spain, 11Rheumatology, University Hospital of Donosti, San Sebastián, Spain, 12Rheumatology, Hospital La Princesa, Madrid, Spain, 13Rheumatology department, Complexo Hospitalario Universitario A Coruña (CHUAC). Instituto de Investigación Biomédica A Coruña (INIBIC), A Coruña, Spain, 14Hospital Universitario Marqués de Valdecilla, IDIVAL, Santander, Spain, 15Department of Rheumatology, University Hospital of Ourense, Ourense, Spain, 16Department of Rheumatology, University Hospital of Alicante, Alicante, Spain, 17Hospital Regional Universitario de Málaga, Malaga, Spain, 18Department of Rheumatology, Hospital Germans Trias i Pujol, Badalona, Spain, 19Severo Ochoa Hospital, Madrid, Spain, 20Hospital Universitario de Bellvitge, Barcelona, Spain, 21Rheumatology, Hospital Jerez, Puerto De Santa María, Spain, 22Department of Rheumatology, Hospital Villajoyosa, Alicante, Spain, 23Rheumatology, Hospital Insular de Gran Canaria, Las Palmas de Gran Canaria, Spain, 24Department of Rheumatology, Hospital Universitario Miguel Servet, Zaragoza, Spain, 25Department of Rheumatology, Hospital Sierrallana, Torrelavega, Spain, 26Galdakao-Usansolo University Hospital, Bilbao, Spain, 27Rheumatology, University Hospital of Salamanca, Salamanca, Spain, 28Department of Rheumatology, Hospital Clínico Universitario La Paz, Madrid, Spain, 29Department of Rheumatology, Hospital de Sant Joan Despí Moises Broggi,, Sant Joan Despí, Spain, 30Department of Rheumatology, Hospital Son Llatzer, Palma de Mallorca, Spain, 31Hospital Ramón y Cajal, Madrid, Spain, 32Rheumatology, Hospital Universitario de León, León, Spain, 33Complejo Universitario de Navarra, Pamplona, Spain, 34Department of Rheumatology, Hospital Clínico Universitario Virgen de la Arrixaca, Murcia, Spain, 35Department of Rheumatology, University Hospital Príncipe de Asturias, Alcalá de Henares, Spain, 36Rheumatology, Hospital do Meixoeiro, Vigo, Spain
Background/Purpose: In systemic lupus erythematosus (SLE), there is no definition of states of moderate and severe SLE activity. How these states may influence different disease outcomes is unknown.
To propose a definition for states of moderate and severe activity in SLE and using the largest Spanish national cohort of SLE patients, to describe the prevalence of both states of activity and to analyze the impact of this classification on flare-ups, achieving low disease activity state (LLDAS), hospital admissions, health-related quality of life (HRQoL), damage accrual and mortality.
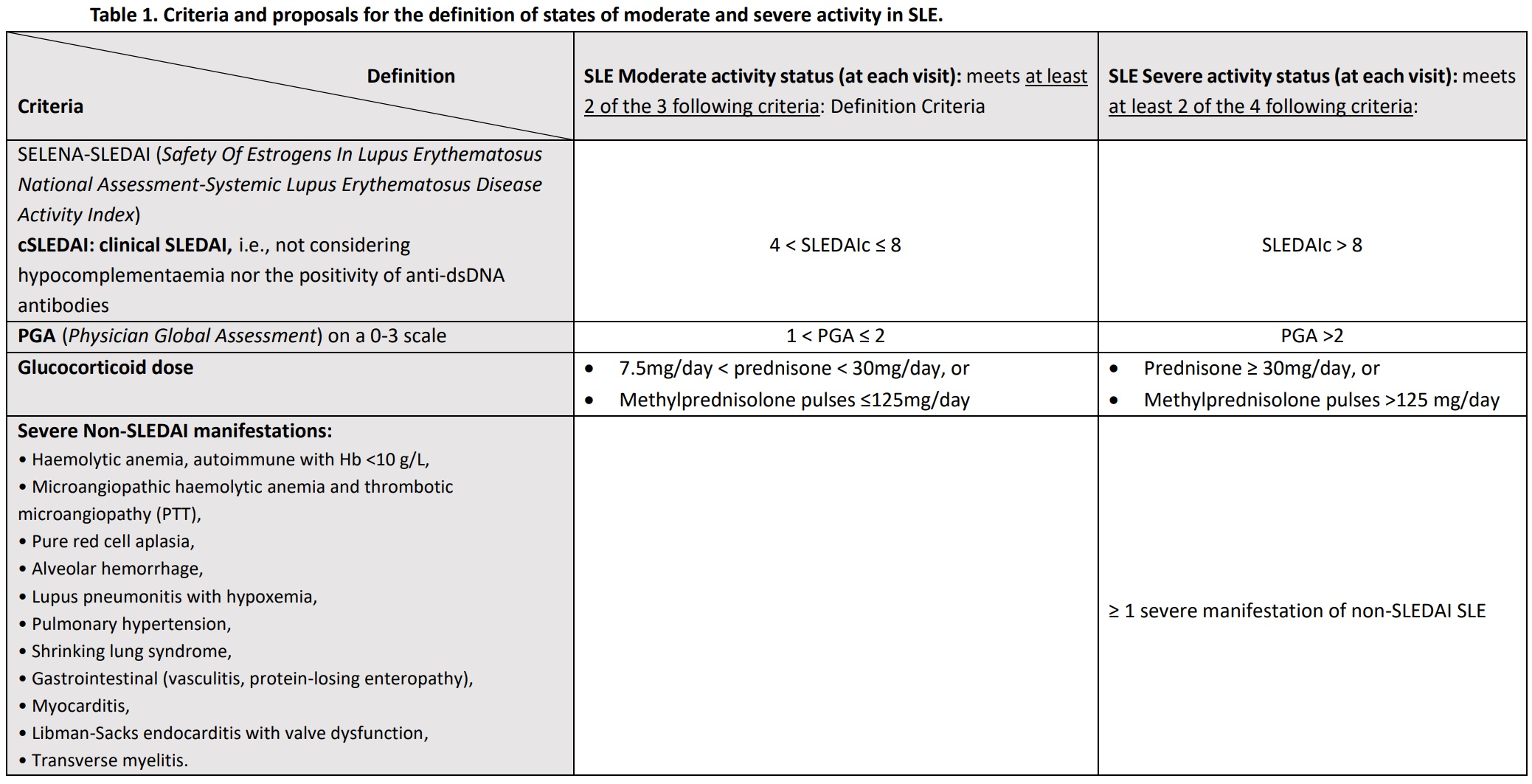
Methods: We propose definitions for states of moderate and severe activity in SLE (Table 1). To analyze the impact of this classification, available data from the prospective phaseof the Spanish Society of Rheumatology SLE Registry (RELESSER-PROS) were used, with 5 annual visits (V1-V5) over 4 years. Patients were required to have at least 3 consecutive visits. At each visit, the number of flares and their severity (according to the SELENA Flare Index), visits in LLDAS, hospital admissions, HRQoL according to the Lupus Impact Tracker (LIT), damage accrual (using the SLICC/ACR Damage Index [SDI]) and mortality was collected. T-test was used for group comparisons.
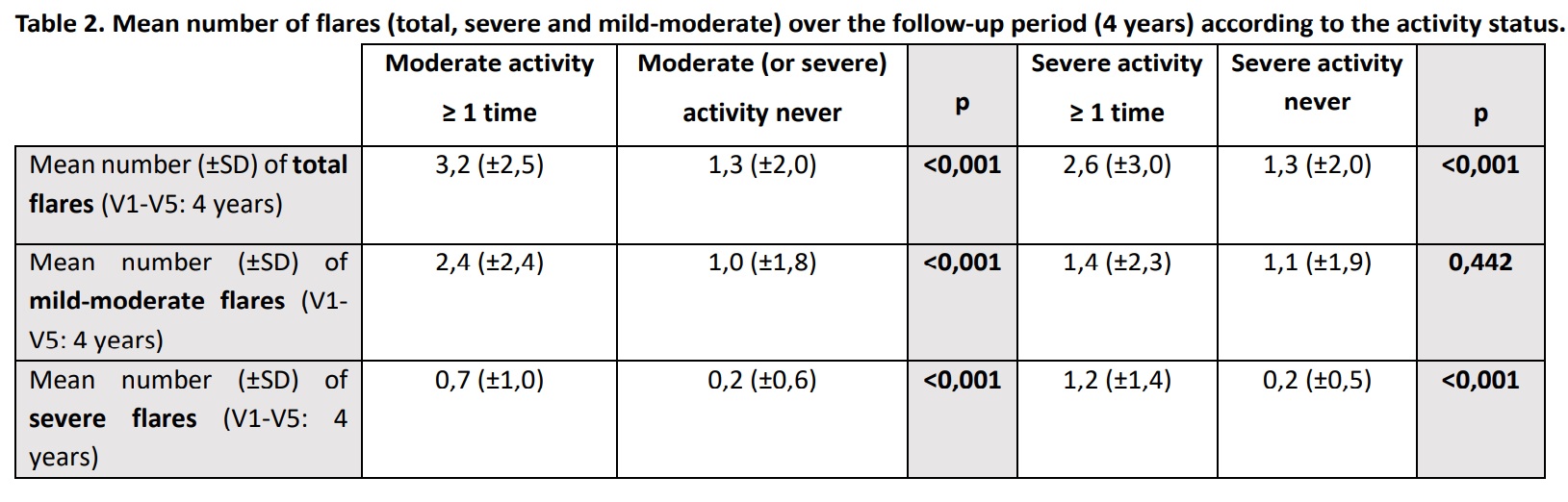
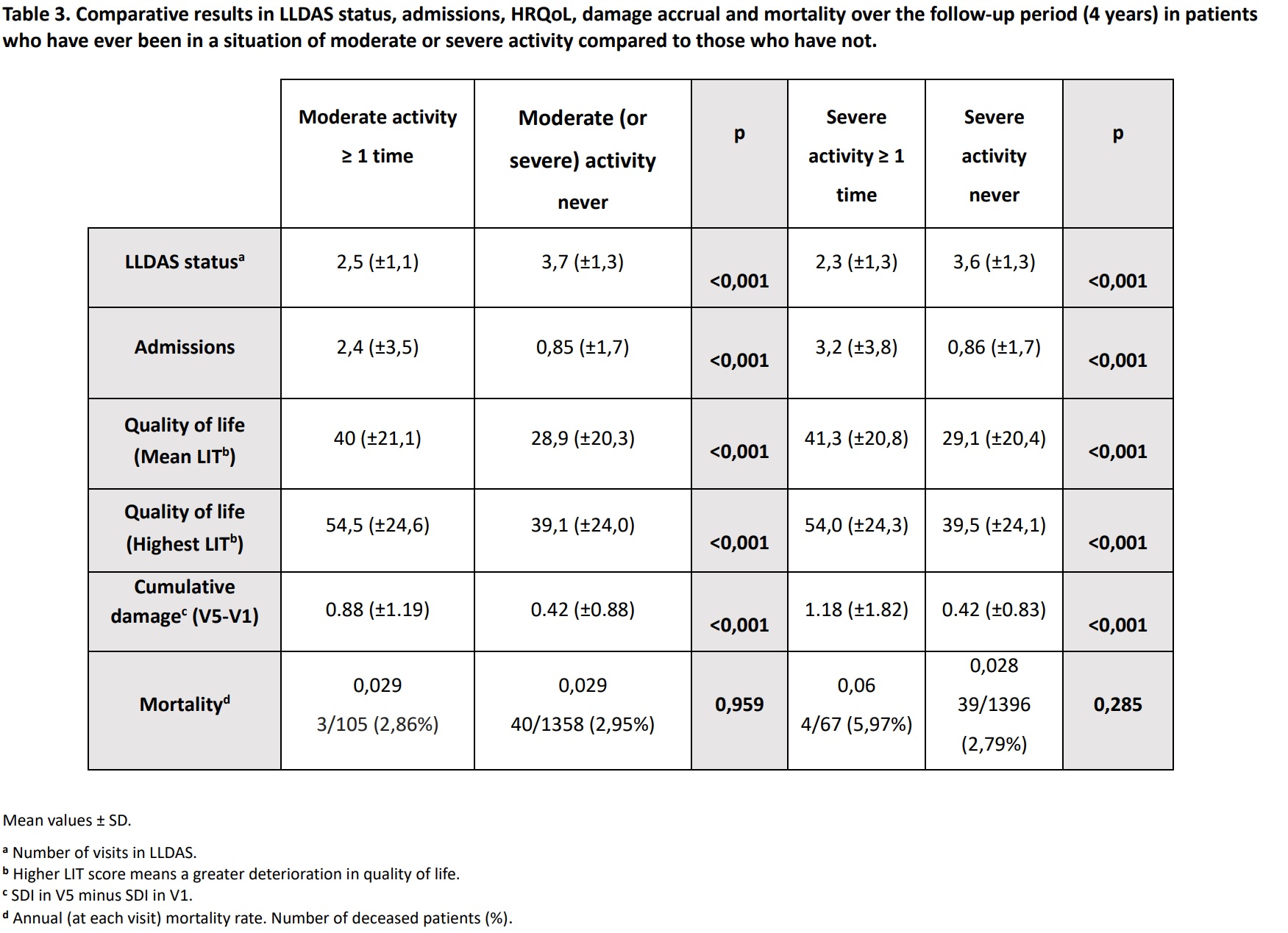
Results: A total of 1463 patients (90% women) were included, with a mean age (±SD) of 56 (±13.5) years. The mean disease duration of SLE (±SD) at V1 was 14 (±8.5) years. Patients had a mean (±SD) of 4.2 (±1.2) visits and a mean (±SD) follow-up of 2.5 (±0.7) years. Moderate activity was shown by 54 patients (3.7%) at V1, 20 patients (1.4%) at V2, 27 patients (1.8%) at V3, 5 patients (0.3%) at V4, and 11 patients (0.8%) at V5. On the other hand, 40 patients (2.7%) at V1, 15 patients (1.0%) at V2, 13 patients (0.9%) at V3, 6 patients (0.4%) at V4 and 3 patients (0.2%) at V5 showed severe activity. Patients who presented both moderate and severe activity in at least 1 of the 5 visits had a significantly higher mean total number of flares over the 4-year follow-up period (V1-V5)than those who did not (p< 0.001). These same results occur in the case of severe flares. Regarding mild-moderate flares, patients with moderate and severe activity in at least 1 visit, had a higher mean number than patients without these activities, being statistically significant for moderate activity. More detailed information about flares can be seen in Table 2. On the other hand, patients who presented both moderate and severe activity in at least 1 of the 5 visits had significantly less number of visits in LLDAS, higher number of hospital admissions, worse HRQoL and greater damage accrual in the period V1-V5 than those who did not (p< 0.001 for all comparisons). Patients with severe activity in ≥1 visit had higher mortality (p=n.s.) (Table 3).
Conclusion: Patients who were in a state of moderate and/or severe activity at least on 1 occasion had worse outcomes at the end of follow-up in terms of number/severity of flares, hospital admissions, deterioration in HRQoL and damage accrual. These results emphasize the importance of setting achievable objectives in the Treat to Target (T2T) strategy for the treatment of SLE.
I. Mamani Velarde: None; I. Rúa-Figueroa: AstraZeneca, 5, GSK, 1, 6; S. García Pérez: None; I. Altabás González: None; C. Mourino Rodriguez: None; N. Jiménez: None; J. MARTINEZ BARRIO: None; M. Galindo-Izquierdo: None; J. Calvo- Alén: AbbVie, 2, AstraZeneca, 2, Biogen, 6, BMS, 5, Galapagos, 6, GSK, 2, 6, Lilly, 2, 6, Novartis, 2, 6, Roche, 5, Sanofi, 2; C. Eurasquin: None; B. Serrano Benavente: None; E. Uriarte Isacelaya: None; E. Tomero Muriel: None; M. Freire González: None; R. Blanco: AbbVie, 5, 6, Amgen, 6, AstraZeneca, 2, BMS, 6, Eli Lilly, 6, Galapagos, 2, 6, Janssen, 2, 6, MSD, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche, 5, 6, Sanofi, 6; E. Salgado-Pérez: None; P. Vela: None; A. Fernandez-Nebro: None; A. Olivé-Marqués: None; C. Sanguesa Gomez: None; J. Narvaez: None; R. Menor-Almagro: None; J. Rosas: None; J. Hernández Beriain: None; J. MANERO: None; E. Aurrecoechea Aguinaga: None; O. Ibarguengoitia-Barrena: None; C. Montilla-Morales: None; G. Bonilla: None; V. Torrente-Segarra: None; A. Cacheda: None; M. García-Villanueva: AstraZeneca, 6, GSK, 6, Otsuka, 1; C. Moriano Morales: None; C. Fito Manteca: None; N. Lozano Rivas: None; C. Bohórquez: None; J. Pego-Reigosa: None.
Background/Purpose: In systemic lupus erythematosus (SLE), there is no definition of states of moderate and severe SLE activity. How these states may influence different disease outcomes is unknown.
To propose a definition for states of moderate and severe activity in SLE and using the largest Spanish national cohort of SLE patients, to describe the prevalence of both states of activity and to analyze the impact of this classification on flare-ups, achieving low disease activity state (LLDAS), hospital admissions, health-related quality of life (HRQoL), damage accrual and mortality.
Methods: We propose definitions for states of moderate and severe activity in SLE (Table 1). To analyze the impact of this classification, available data from the prospective phaseof the Spanish Society of Rheumatology SLE Registry (RELESSER-PROS) were used, with 5 annual visits (V1-V5) over 4 years. Patients were required to have at least 3 consecutive visits. At each visit, the number of flares and their severity (according to the SELENA Flare Index), visits in LLDAS, hospital admissions, HRQoL according to the Lupus Impact Tracker (LIT), damage accrual (using the SLICC/ACR Damage Index [SDI]) and mortality was collected. T-test was used for group comparisons.
Results: A total of 1463 patients (90% women) were included, with a mean age (±SD) of 56 (±13.5) years. The mean disease duration of SLE (±SD) at V1 was 14 (±8.5) years. Patients had a mean (±SD) of 4.2 (±1.2) visits and a mean (±SD) follow-up of 2.5 (±0.7) years. Moderate activity was shown by 54 patients (3.7%) at V1, 20 patients (1.4%) at V2, 27 patients (1.8%) at V3, 5 patients (0.3%) at V4, and 11 patients (0.8%) at V5. On the other hand, 40 patients (2.7%) at V1, 15 patients (1.0%) at V2, 13 patients (0.9%) at V3, 6 patients (0.4%) at V4 and 3 patients (0.2%) at V5 showed severe activity. Patients who presented both moderate and severe activity in at least 1 of the 5 visits had a significantly higher mean total number of flares over the 4-year follow-up period (V1-V5)than those who did not (p< 0.001). These same results occur in the case of severe flares. Regarding mild-moderate flares, patients with moderate and severe activity in at least 1 visit, had a higher mean number than patients without these activities, being statistically significant for moderate activity. More detailed information about flares can be seen in Table 2. On the other hand, patients who presented both moderate and severe activity in at least 1 of the 5 visits had significantly less number of visits in LLDAS, higher number of hospital admissions, worse HRQoL and greater damage accrual in the period V1-V5 than those who did not (p< 0.001 for all comparisons). Patients with severe activity in ≥1 visit had higher mortality (p=n.s.) (Table 3).
Conclusion: Patients who were in a state of moderate and/or severe activity at least on 1 occasion had worse outcomes at the end of follow-up in terms of number/severity of flares, hospital admissions, deterioration in HRQoL and damage accrual. These results emphasize the importance of setting achievable objectives in the Treat to Target (T2T) strategy for the treatment of SLE.

Table 1. Criteria and proposals for the definition of states of moderate and severe activity in SLE.

Table 2. Mean number of flares (total, severe and mild-moderate) over the follow-up period (4 years) according to the activity status.

Table 3. Comparative results in LLDAS status, admissions, HRQoL, damage accrual and mortality over the follow-up period (4 years) in patients
who have ever been in a situation of moderate or severe activity compared to those who have not.
who have ever been in a situation of moderate or severe activity compared to those who have not.
I. Mamani Velarde: None; I. Rúa-Figueroa: AstraZeneca, 5, GSK, 1, 6; S. García Pérez: None; I. Altabás González: None; C. Mourino Rodriguez: None; N. Jiménez: None; J. MARTINEZ BARRIO: None; M. Galindo-Izquierdo: None; J. Calvo- Alén: AbbVie, 2, AstraZeneca, 2, Biogen, 6, BMS, 5, Galapagos, 6, GSK, 2, 6, Lilly, 2, 6, Novartis, 2, 6, Roche, 5, Sanofi, 2; C. Eurasquin: None; B. Serrano Benavente: None; E. Uriarte Isacelaya: None; E. Tomero Muriel: None; M. Freire González: None; R. Blanco: AbbVie, 5, 6, Amgen, 6, AstraZeneca, 2, BMS, 6, Eli Lilly, 6, Galapagos, 2, 6, Janssen, 2, 6, MSD, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche, 5, 6, Sanofi, 6; E. Salgado-Pérez: None; P. Vela: None; A. Fernandez-Nebro: None; A. Olivé-Marqués: None; C. Sanguesa Gomez: None; J. Narvaez: None; R. Menor-Almagro: None; J. Rosas: None; J. Hernández Beriain: None; J. MANERO: None; E. Aurrecoechea Aguinaga: None; O. Ibarguengoitia-Barrena: None; C. Montilla-Morales: None; G. Bonilla: None; V. Torrente-Segarra: None; A. Cacheda: None; M. García-Villanueva: AstraZeneca, 6, GSK, 6, Otsuka, 1; C. Moriano Morales: None; C. Fito Manteca: None; N. Lozano Rivas: None; C. Bohórquez: None; J. Pego-Reigosa: None.



