Poster Session C
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (2195–2226) Spondyloarthritis Including Psoriatic Arthritis – Diagnosis, Manifestations, & Outcomes Poster III: SpA
2201: When Usual Care Is Not so Usual: Facing the Challenges of Protocol Violations and Generalisability When Running a Strategy Trial. the Example of the Cluster-Randomized TICOSPA Trial
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
.jpg)
Clementina Lopez-Medina, MD
Reina Sofia University Hospital
Cordoba, SpainDisclosure information not submitted.
Abstract Poster Presenter(s)
Clementina López Medina1, Filip Van den Bosch2, Désirée van der Heijde3, Maxime Dougados4 and Anna Molto5, 1Rheumatology Department, Cochin Hospital; INSERM (U1153): Clinical Epidemiology and Biostatistics, University of Paris; Rheumatology Department, Reina Sofia Hospital, Cordoba / IMIBIC / University of Cordoba, Cordoba, Spain, 2Department of Internal Medicine and Pediatrics, Ghent University and VIB Center for Inflammation Research, Ghent, Belgium, 3Department of Rheumatology, Leiden University Medical Center, Leiden, Netherlands, 4Rheumatology Department, Cochin Hospital; INSERM (U1153): Clinical Epidemiology and Biostatistics, University of Paris, Paris, France, 5HOPITAL COCHIN AP-HP, Service de Rhumatologie, Paris, France
Background/Purpose: Despite the ASAS-HI (primary outcome) did not reach statistical significance in the TICOSPA trial, other secondary outcomes were numerically higher in the treat-to-target (T2T) strategy in comparison to Usual Care (UC). Three hypotheses have been considered to explain this: a lack of power, the risk of protocol violations in the T2T arm and the potential optimal care in the UC arm. Objectives: a) to evaluate the proportion of patients (pts) who violated the protocol in the T2T arm during the 48 weeks (48W) of follow up as well as the impact and predictive factors of such violation; b) to compare the proportion of pts treated according to the ASAS/EULAR 2016 management recommendations for axSpA over the first 12W of follow-up period in both arms.
Methods: Study design: pragmatic, prospective, cluster-randomized controlled, 48W trial (NCT03043846). Inclusion criteria: Pts with a diagnosis of axSpA and fulfilling ASAS criteria, non-optimally treated with NSAIDs, bDMARD-naïve and ASDAS >2.1. Study treatment regimens:SpA expert centers were randomly allocated (1:1) to the treatment arm: a) T2T: pre-specified management strategy (Q4W visits), with a target of ASDAS< 2.1; b) UC: treatment decisions at the rheumatologist's discretion (Q12W). Outcome: Protocol violations in the T2T arm were evaluated at every visit by a specific question. Statistical analysis: Factors associated with at least one protocol violation over the study were evaluated using multivariate logistic regression. Outcomes at 48W were compared between T2T violators (T2T-V) vs. T2T non-violators (T2T-NV) vs. UC using GEE models adjusted by country and sex; b) optimal care in UC: proportion of pts treated according to the 2016 ASAS/EULAR recommendations over the first 12W in both arms were compared.
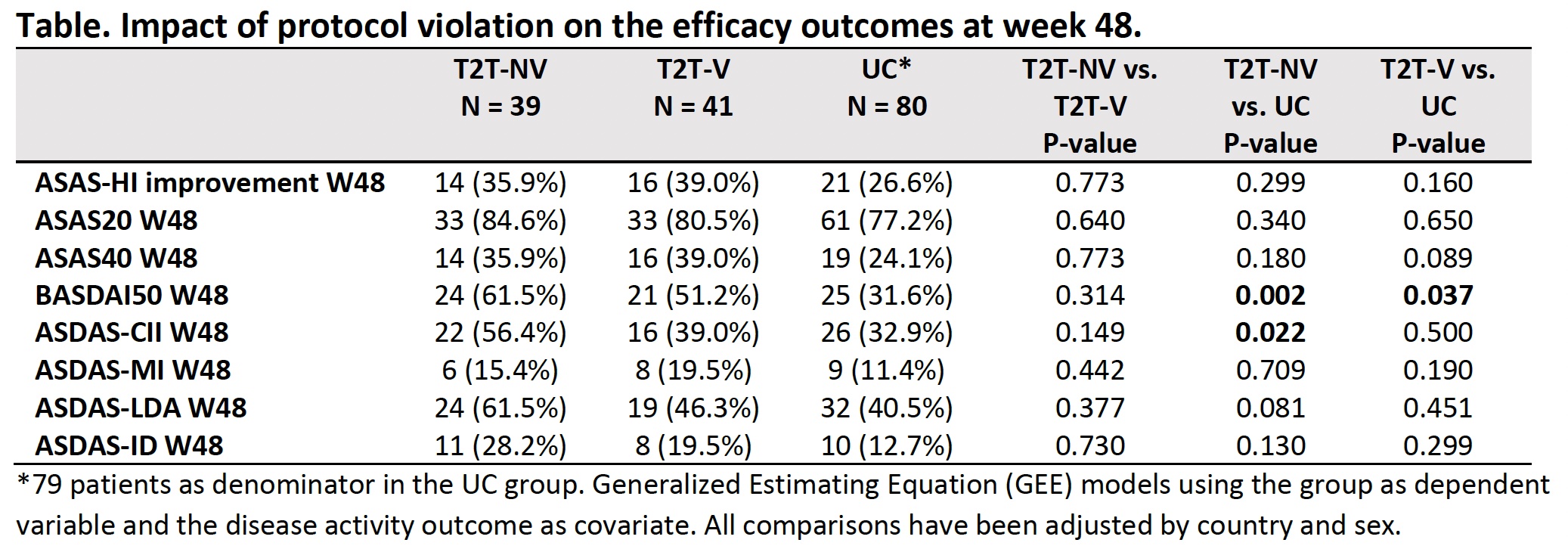
Results: 160 pts initiated the trial (T2T:80 and UC:80). a) Protocol violations: In the T2T arm, 41/80 (51.2%) pts violated the protocol during at least one visit, with a total of 119 violations (27.7% represented by an intensification of bDMARD and 62.2% by a maintenance or reduction of the treatment against protocol). Baseline predictive factors independently associated with the protocol violation were the country (France vs. others; OR 3.8 (95%CI 1.1-15.0)), female sex (OR 4.4 (1.5-15.1)), diagnosis delay ≤7 years (OR 3.4 (1.1-11.9)), HLA-B27 negative (OR 6.4 (1.6-32.2)) and CRP≥6mg/L (OR 4.2 (1.3-15.9)). After 48W of follow-up, T2T-NV vs. T2T-V showed similar ratios of ASAS-HI improvement. ASDAS-LDA, ASDAS-ID and ASDAS-CII outcomes were more prevalent in T2T-NV vs. T2T-V, although they did not reach statistical significance (Table). b) Optimal care in UC: the proportion of pts managed according to the 2016 ASAS/EULAR recommendations during the first 12W of follow-up was similar in both arms (63.9% in T2T vs. 61.8% in UC, p=0.490) (Figure).
Conclusion: These results confirm that protocol violations in the T2T arm in the TICOSPA trial were frequent, although they did not have an impact on the rate of the primary outcome. The proportion of patients managed according to the 2016 ASAS/EULAR recommendations in the UC arm was very high, suggesting that the UC group was optimally treated and explaining the non-achievement of the primary objective in the TICOSPA trial.
.jpg)
C. López Medina: AbbVie, 5, 6, Eli Lilly, 2, 5, 6, Janssen, 6, MSD, 6, Novartis, 2, 5, 6, UCB Pharma, 2, 5, 6; F. Van den Bosch: AbbVie, 2, 6, Amgen, 2, BMS, 6, Celgene, 6, Eli Lilly, 2, Galapagos, 2, Janssen, 2, 6, Merck, 2, 6, Novartis, 2, 6, Pfizer, 2, 6, UCB Pharma, 2, 6; D. van der Heijde: AbbVie, 2, Bayer, 2, BMS, 2, Eli Lilly, 2, Galapagos, 2, Gilead, 2, GSK, 2, Imaging Rheumatology BV, 12, Director, Janssen, 2, Novartis, 2, Pfizer, 2, Takeda, 2, UCB Pharma, 2; M. Dougados: AbbVie, 2, 5, Bristol Myers Squibb, 2, 5, Eli Lilly, 2, 5, MSD, 2, 5, Novartis, 2, 5, Pfizer Inc, 2, 5, Roche, 2, 5, UCB, 2, 5; A. Molto: None.
Background/Purpose: Despite the ASAS-HI (primary outcome) did not reach statistical significance in the TICOSPA trial, other secondary outcomes were numerically higher in the treat-to-target (T2T) strategy in comparison to Usual Care (UC). Three hypotheses have been considered to explain this: a lack of power, the risk of protocol violations in the T2T arm and the potential optimal care in the UC arm. Objectives: a) to evaluate the proportion of patients (pts) who violated the protocol in the T2T arm during the 48 weeks (48W) of follow up as well as the impact and predictive factors of such violation; b) to compare the proportion of pts treated according to the ASAS/EULAR 2016 management recommendations for axSpA over the first 12W of follow-up period in both arms.
Methods: Study design: pragmatic, prospective, cluster-randomized controlled, 48W trial (NCT03043846). Inclusion criteria: Pts with a diagnosis of axSpA and fulfilling ASAS criteria, non-optimally treated with NSAIDs, bDMARD-naïve and ASDAS >2.1. Study treatment regimens:SpA expert centers were randomly allocated (1:1) to the treatment arm: a) T2T: pre-specified management strategy (Q4W visits), with a target of ASDAS< 2.1; b) UC: treatment decisions at the rheumatologist's discretion (Q12W). Outcome: Protocol violations in the T2T arm were evaluated at every visit by a specific question. Statistical analysis: Factors associated with at least one protocol violation over the study were evaluated using multivariate logistic regression. Outcomes at 48W were compared between T2T violators (T2T-V) vs. T2T non-violators (T2T-NV) vs. UC using GEE models adjusted by country and sex; b) optimal care in UC: proportion of pts treated according to the 2016 ASAS/EULAR recommendations over the first 12W in both arms were compared.
Results: 160 pts initiated the trial (T2T:80 and UC:80). a) Protocol violations: In the T2T arm, 41/80 (51.2%) pts violated the protocol during at least one visit, with a total of 119 violations (27.7% represented by an intensification of bDMARD and 62.2% by a maintenance or reduction of the treatment against protocol). Baseline predictive factors independently associated with the protocol violation were the country (France vs. others; OR 3.8 (95%CI 1.1-15.0)), female sex (OR 4.4 (1.5-15.1)), diagnosis delay ≤7 years (OR 3.4 (1.1-11.9)), HLA-B27 negative (OR 6.4 (1.6-32.2)) and CRP≥6mg/L (OR 4.2 (1.3-15.9)). After 48W of follow-up, T2T-NV vs. T2T-V showed similar ratios of ASAS-HI improvement. ASDAS-LDA, ASDAS-ID and ASDAS-CII outcomes were more prevalent in T2T-NV vs. T2T-V, although they did not reach statistical significance (Table). b) Optimal care in UC: the proportion of pts managed according to the 2016 ASAS/EULAR recommendations during the first 12W of follow-up was similar in both arms (63.9% in T2T vs. 61.8% in UC, p=0.490) (Figure).
Conclusion: These results confirm that protocol violations in the T2T arm in the TICOSPA trial were frequent, although they did not have an impact on the rate of the primary outcome. The proportion of patients managed according to the 2016 ASAS/EULAR recommendations in the UC arm was very high, suggesting that the UC group was optimally treated and explaining the non-achievement of the primary objective in the TICOSPA trial.

.jpg)
Figure. Proportion of patients treated according to the ASAS/EULAR 2016 management recommendations for axSpA in both groups during the first 12 weeks of follow-up.
C. López Medina: AbbVie, 5, 6, Eli Lilly, 2, 5, 6, Janssen, 6, MSD, 6, Novartis, 2, 5, 6, UCB Pharma, 2, 5, 6; F. Van den Bosch: AbbVie, 2, 6, Amgen, 2, BMS, 6, Celgene, 6, Eli Lilly, 2, Galapagos, 2, Janssen, 2, 6, Merck, 2, 6, Novartis, 2, 6, Pfizer, 2, 6, UCB Pharma, 2, 6; D. van der Heijde: AbbVie, 2, Bayer, 2, BMS, 2, Eli Lilly, 2, Galapagos, 2, Gilead, 2, GSK, 2, Imaging Rheumatology BV, 12, Director, Janssen, 2, Novartis, 2, Pfizer, 2, Takeda, 2, UCB Pharma, 2; M. Dougados: AbbVie, 2, 5, Bristol Myers Squibb, 2, 5, Eli Lilly, 2, 5, MSD, 2, 5, Novartis, 2, 5, Pfizer Inc, 2, 5, Roche, 2, 5, UCB, 2, 5; A. Molto: None.



