Poster Session B
Reproductive health
Session: (1345–1364) Reproductive Issues in Rheumatic Disorders Poster II
1346: Semen Analysis of Patients with Psoriatic Arthritis, Axial Spondyloarthritis and Healthy Controls
Monday, November 13, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- KM
Abstract Poster Presenter(s)
Katya Meridor1, Shimi Barda2, Ofir Elalouf2, Victoria Furer2, Sara Pel3, Hila Nochomovitz4, michael zisapel2, Jonathan Wollman2, Reut Tzemach2, Mark Berman5, Sara Borok6, Hagit Sarbagil-Maman7, Hagit Padova8, David levartovsky2, Tzippy Shochat9, Daphna Paran2, Ron Hauser2, Ori Elkayam2 and Ari Polachek6, 1Tel Aviv Medical Center, Kfar Saba, Israel, 2Tel Aviv Medical Center, Tel Aviv, Israel, 3Tel Aviv Medical Center, Modiin, Israel, 4Ichilov, Tel Aviv, Israel, 5Tel Aviv Sourasky Medical Center, Tel Aviv, Israel, 6Sourasky Medical Center, Tel Aviv, Israel, 7Tel Aviv Sourasky Medical center, Kiryat Ono, Israel, 8Tel Aviv Medical center, Petah-Tikva, Israel, 9Rabin Medical Center, Modiin, Israel
Background/Purpose: Psoriatic arthritis (PsA) and axial spondyloarthritis (AxSpA) are commonly diagnosed in young males in their reproductive years. However, only a few studies have investigated male fertility in patients with spondyloarthritis (SpA). Our objective was to evaluate the sperm quality in male patients with PsA and AxSpA compared to healthy controls (HC) and to investigate the effects of disease activity and anti-rheumatic drugs on sperm quality.
Methods: Consecutive PsA and AxSpA patients (age range 18-50 years), who fulfilled the classification criteria for PsA (CASPAR) and AxSpA (ASAS) were recruited prospectively. HC were recruited from candidate sperm donors at the male fertility clinic. Each patient was evaluated by a comprehensive clinical assessment that included measurement of disease activity scores (MDA/DAPSA/CPDAI/PASDAS for PsA and ASDAS for AxSpA). Treatments ranged from no treatment to conventional and biologic DMARDs. Sperm collection and analysis were performed on the day of clinical assessment. Sperm analysis was performed according to the World Health Organization 2010 guidelines. In addition, sperm DNA-fragmentation test was performed.
Continuous variables were compared using a t-test or Anova for normally distributed data, otherwise, a non-parametric test was applied. Fisher's exact test was used to compare categorical variables.
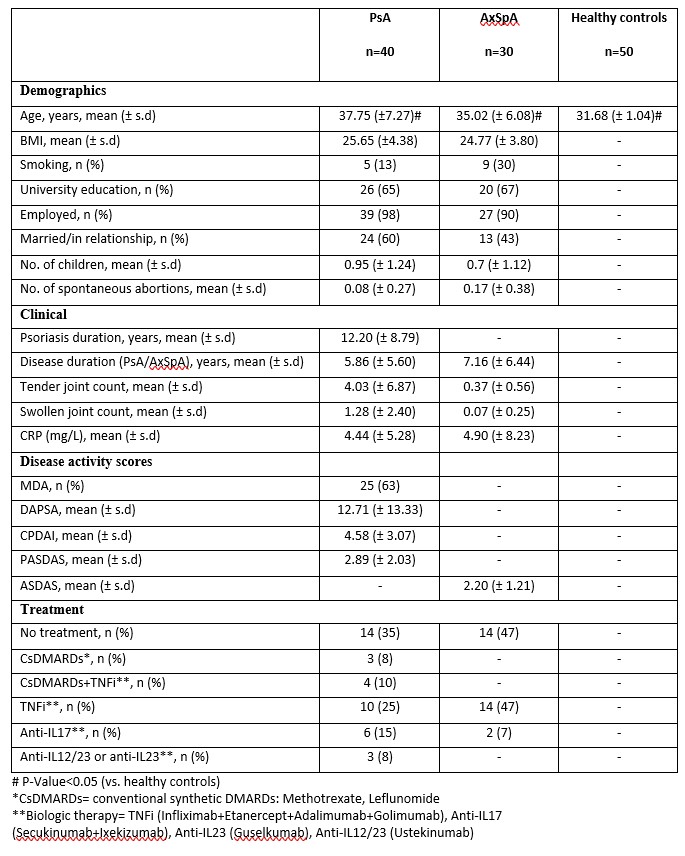
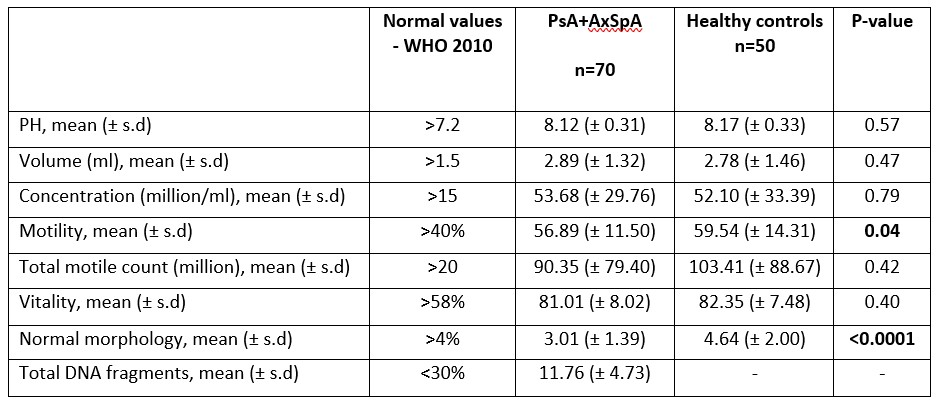
Results: 70 patients (40 PsA and 30 AxSpA) and 50 HC were included. Demographics and clinical characteristics are presented in Table 1. Most semen parameters, including concentration, and vitality of PsA and AxSpA patients were similar to those of HC (p >0.05).The proportion of normal morphology in patients was significantly lower compared to HC (3.01 (± 1.39) vs 4.64 (±2.00), p< 0.0001). The motility in patients vs. controls was lower (56.89 (± 11.50) vs. 59.54 (± 14.31), p=0.04). Nevertheless, values for both groups were within the normal range ( >40%) and the total motile count was normal and similar between the groups (Table 2).
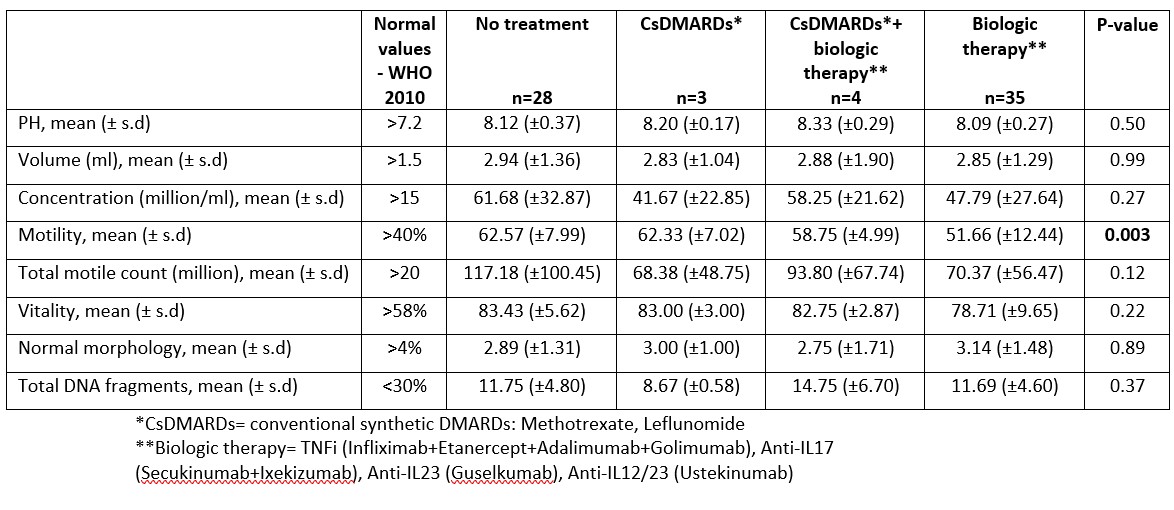
In addition, no differences were observed in semen analysis in the different disease activity states (remission/low and moderate/high disease activity) for PsA and for AxSpA (p >0.05). Finally, a comparison of the different treatment options (no treatment, conventional and biologic DMARDs) showed overall no differences, while motility was slightly lower in patients treated with biologic therapy (62.57 (±7.99), 62.33 (±7.02), 58.75 (±4.99) and 51.66 (±12.44) for no treatment, conventional synthetic DMARDs, conventional synthetic DMARDs+biologic therapy, and biologic monotherapy respectively, p=0.003). However, values for all groups were within the normal range ( >40%) and the total motile count was normal and similar between the groups (Table 3).
Conclusion: In this relatively large study evaluating semen analysis in spondyloarthropathies, the semen quality of PsA and AxSpA patients was comparable to HC in most parameters. In addition, neither disease activity nor antirheumatic drugs substantially affected sperm quality. Our results do not support cryopreservation of semen before treatment initiation nor a drug free interval prior to conception. Further longitudinal large studies are needed.
K. Meridor: None; S. Barda: None; O. Elalouf: None; V. Furer: None; S. Pel: None; H. Nochomovitz: None; m. zisapel: None; J. Wollman: None; R. Tzemach: None; M. Berman: None; S. Borok: None; H. Sarbagil-Maman: None; H. Padova: None; D. levartovsky: None; T. Shochat: None; D. Paran: None; R. Hauser: None; O. Elkayam: None; A. Polachek: None.
Background/Purpose: Psoriatic arthritis (PsA) and axial spondyloarthritis (AxSpA) are commonly diagnosed in young males in their reproductive years. However, only a few studies have investigated male fertility in patients with spondyloarthritis (SpA). Our objective was to evaluate the sperm quality in male patients with PsA and AxSpA compared to healthy controls (HC) and to investigate the effects of disease activity and anti-rheumatic drugs on sperm quality.
Methods: Consecutive PsA and AxSpA patients (age range 18-50 years), who fulfilled the classification criteria for PsA (CASPAR) and AxSpA (ASAS) were recruited prospectively. HC were recruited from candidate sperm donors at the male fertility clinic. Each patient was evaluated by a comprehensive clinical assessment that included measurement of disease activity scores (MDA/DAPSA/CPDAI/PASDAS for PsA and ASDAS for AxSpA). Treatments ranged from no treatment to conventional and biologic DMARDs. Sperm collection and analysis were performed on the day of clinical assessment. Sperm analysis was performed according to the World Health Organization 2010 guidelines. In addition, sperm DNA-fragmentation test was performed.
Continuous variables were compared using a t-test or Anova for normally distributed data, otherwise, a non-parametric test was applied. Fisher's exact test was used to compare categorical variables.
Results: 70 patients (40 PsA and 30 AxSpA) and 50 HC were included. Demographics and clinical characteristics are presented in Table 1. Most semen parameters, including concentration, and vitality of PsA and AxSpA patients were similar to those of HC (p >0.05).The proportion of normal morphology in patients was significantly lower compared to HC (3.01 (± 1.39) vs 4.64 (±2.00), p< 0.0001). The motility in patients vs. controls was lower (56.89 (± 11.50) vs. 59.54 (± 14.31), p=0.04). Nevertheless, values for both groups were within the normal range ( >40%) and the total motile count was normal and similar between the groups (Table 2).
In addition, no differences were observed in semen analysis in the different disease activity states (remission/low and moderate/high disease activity) for PsA and for AxSpA (p >0.05). Finally, a comparison of the different treatment options (no treatment, conventional and biologic DMARDs) showed overall no differences, while motility was slightly lower in patients treated with biologic therapy (62.57 (±7.99), 62.33 (±7.02), 58.75 (±4.99) and 51.66 (±12.44) for no treatment, conventional synthetic DMARDs, conventional synthetic DMARDs+biologic therapy, and biologic monotherapy respectively, p=0.003). However, values for all groups were within the normal range ( >40%) and the total motile count was normal and similar between the groups (Table 3).
Conclusion: In this relatively large study evaluating semen analysis in spondyloarthropathies, the semen quality of PsA and AxSpA patients was comparable to HC in most parameters. In addition, neither disease activity nor antirheumatic drugs substantially affected sperm quality. Our results do not support cryopreservation of semen before treatment initiation nor a drug free interval prior to conception. Further longitudinal large studies are needed.

Table 1 - Demographic and clinical characteristics of patients with PsA, AxSpA and healthy controls

Table 2 - Semen analysis of patients with PsA and AxSpA compared with healthy controls

K. Meridor: None; S. Barda: None; O. Elalouf: None; V. Furer: None; S. Pel: None; H. Nochomovitz: None; m. zisapel: None; J. Wollman: None; R. Tzemach: None; M. Berman: None; S. Borok: None; H. Sarbagil-Maman: None; H. Padova: None; D. levartovsky: None; T. Shochat: None; D. Paran: None; R. Hauser: None; O. Elkayam: None; A. Polachek: None.



