Poster Session C
Rheumatoid arthritis (RA)
Session: (2141–2176) RA – Treatment Poster III
2146: After JAK Inhibitor Failure, "Switching" or "Cycling"?
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- PM
Pablo Francisco Muñoz Martínez, MD
Hospital Universitario y Politécnico La Fe
Sagunto, SpainDisclosure information not submitted.
Abstract Poster Presenter(s)
Pablo Francisco Muñoz Martínez1, Laura Mas Sanchez2, Elena Grau Garcia3, Carmen Riesco Barcena3, Anderson Victor Huaylla Quispe3, Alba Maria Torrat Noves3, Daniel Ramos Castro3, Belen Villanueva Mañez3, Iago Alcantara Alvarez3, Samuel Leal Rodriguez3, Elvira Vicens Bernabeu3, Jose Eloy Oller Rodriguez3, Ines Canovas Olmos3, Ernesto Tovar Sugrañes3, Carmen Najera Herranz3, Isabel Martinez Cordellat3, Hikmat Charia3, Rosa Negueroles Albuixech3, Marta De la Rubia Navarro2, Luis Gonzalez Puig3, Jose Ivorra Cortes3 and Jose Andres Roman Ivorra4, 1Rheumatology Department. HUP La Fe, Sagunto, Spain, 2Rheumatology Department. HUP La Fe, Valéncia, Spain, 3Rheumatology Department. HUP La Fe, Valencia, Spain, 4Hospital Universitari i Politècnic la Fe, Valencia, Spain
Background/Purpose: The appearance of JAK inhibitors (JAKi) in the last few years has proved a great clinical application in rheumatic pathology and it has become an innovative and widely-used therapeutic line. In fact, JAKi has been recently included in the management algorithms of psoriatic arthritis, axial spondyloarthritis or rheumatoid arthritis. Nevertheless, the evidence of therapeutic alternatives in real life after the failure of a JAK inhibitor is limited: another JAKi or the change in therapeutic target.
The purpose of the study is to analyze the treatment alternatives after JAKi failure in real life conditions and in multiple diseases.
Methods: Retrospective longitudinal observational study of patients that started JAKi treatment and was discontinued from 2013 to 2022. Demographic and clinical variables were collected from the electronic medical record. "Switching" and "cycling" were analyzed depending on the prescribed medicine after the JAKi, classifying them in the following targets: anti-TNF, anti-IL6, anti-CTLA4, anti-IL12/23, anti-IL17, anti-IL23, another JAKi or other situations like anti-CD20. We studied the retention rate at 9 months after the start of the new treatment.
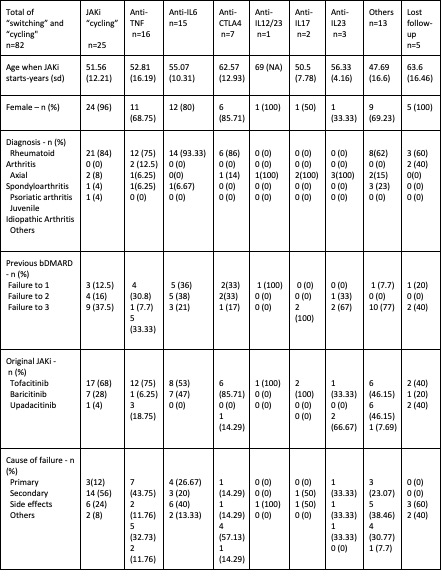
Results: In our series, the most prescribed JAKi was Tofactinib (n=119), followed by Baricitinib (n=76), Upadacitinib (n=66) and Filgotinib (n=4). The most recurrent indications for JAKi treatment were rheumatoid arthritis (n=71) and psoriatic arthritis (n=12).
Out of 265 JAKi prescriptions, 95 failures to JAKi treatment were included in this study. The JAKi with higher failure rate was tofacitinib (n=61) followed by baricitinib (n=22) and upadacitinib (n=12). We do not have data from filgotinib due to its recent incorporation to the Spanish national market. The main cause of end of treatment was adverse reaction (34.74%), followed by secondary failure (28.42%).
After all the JAKi failures, "switching" to other families like anti-TNF or anti-IL6 was the main therapeutical choice (n=57), followed by "cycling" to another JAKi (n=25). 8 patients did not start a new treatment after JAKI failure.
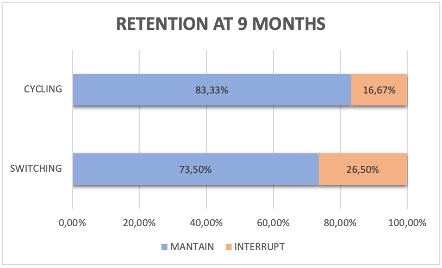
The retention rate at 9 moths after the treatment showed that the 83,33% of the "cycling" to another JAKi and the 73,5% of the "switching" to another therapeutic target maintained the treatment (see image). The retention rate was not studied in 17 patients because the treatment period was less than 9 months.
Conclusion: After a JAKi failure, "switching" is the most recurrent alternative in our series, used most frequently in patients with side events or primary failure. "Cycling" was the most common alternative used with patients who stopped receiving treatment due to secondary failure and in patients with a major proportion of failure to bDMARD.
The retention rate after 9 months was 83,33% for "cycling" and 73,50% for "switching" groups.
P. Muñoz Martínez: None; L. Mas Sanchez: None; E. Grau Garcia: None; C. Riesco Barcena: None; A. Huaylla Quispe: None; A. Torrat Noves: None; D. Ramos Castro: None; B. Villanueva Mañez: None; I. Alcantara Alvarez: None; S. Leal Rodriguez: None; E. Vicens Bernabeu: None; J. Oller Rodriguez: None; I. Canovas Olmos: None; E. Tovar Sugrañes: None; C. Najera Herranz: None; I. Martinez Cordellat: None; H. Charia: None; R. Negueroles Albuixech: None; M. De la Rubia Navarro: None; L. Gonzalez Puig: None; J. Ivorra Cortes: None; J. Roman Ivorra: None.
Background/Purpose: The appearance of JAK inhibitors (JAKi) in the last few years has proved a great clinical application in rheumatic pathology and it has become an innovative and widely-used therapeutic line. In fact, JAKi has been recently included in the management algorithms of psoriatic arthritis, axial spondyloarthritis or rheumatoid arthritis. Nevertheless, the evidence of therapeutic alternatives in real life after the failure of a JAK inhibitor is limited: another JAKi or the change in therapeutic target.
The purpose of the study is to analyze the treatment alternatives after JAKi failure in real life conditions and in multiple diseases.
Methods: Retrospective longitudinal observational study of patients that started JAKi treatment and was discontinued from 2013 to 2022. Demographic and clinical variables were collected from the electronic medical record. "Switching" and "cycling" were analyzed depending on the prescribed medicine after the JAKi, classifying them in the following targets: anti-TNF, anti-IL6, anti-CTLA4, anti-IL12/23, anti-IL17, anti-IL23, another JAKi or other situations like anti-CD20. We studied the retention rate at 9 months after the start of the new treatment.
Results: In our series, the most prescribed JAKi was Tofactinib (n=119), followed by Baricitinib (n=76), Upadacitinib (n=66) and Filgotinib (n=4). The most recurrent indications for JAKi treatment were rheumatoid arthritis (n=71) and psoriatic arthritis (n=12).
Out of 265 JAKi prescriptions, 95 failures to JAKi treatment were included in this study. The JAKi with higher failure rate was tofacitinib (n=61) followed by baricitinib (n=22) and upadacitinib (n=12). We do not have data from filgotinib due to its recent incorporation to the Spanish national market. The main cause of end of treatment was adverse reaction (34.74%), followed by secondary failure (28.42%).
After all the JAKi failures, "switching" to other families like anti-TNF or anti-IL6 was the main therapeutical choice (n=57), followed by "cycling" to another JAKi (n=25). 8 patients did not start a new treatment after JAKI failure.
The retention rate at 9 moths after the treatment showed that the 83,33% of the "cycling" to another JAKi and the 73,5% of the "switching" to another therapeutic target maintained the treatment (see image). The retention rate was not studied in 17 patients because the treatment period was less than 9 months.
Conclusion: After a JAKi failure, "switching" is the most recurrent alternative in our series, used most frequently in patients with side events or primary failure. "Cycling" was the most common alternative used with patients who stopped receiving treatment due to secondary failure and in patients with a major proportion of failure to bDMARD.
The retention rate after 9 months was 83,33% for "cycling" and 73,50% for "switching" groups.

Table 1. Total of "switching" and "cycling"

Image 1. Retention rate at 9 months
P. Muñoz Martínez: None; L. Mas Sanchez: None; E. Grau Garcia: None; C. Riesco Barcena: None; A. Huaylla Quispe: None; A. Torrat Noves: None; D. Ramos Castro: None; B. Villanueva Mañez: None; I. Alcantara Alvarez: None; S. Leal Rodriguez: None; E. Vicens Bernabeu: None; J. Oller Rodriguez: None; I. Canovas Olmos: None; E. Tovar Sugrañes: None; C. Najera Herranz: None; I. Martinez Cordellat: None; H. Charia: None; R. Negueroles Albuixech: None; M. De la Rubia Navarro: None; L. Gonzalez Puig: None; J. Ivorra Cortes: None; J. Roman Ivorra: None.



