Poster Session C
Metabolic bone disease
Session: (1996–2018) Osteoporosis & Metabolic Bone Disease – Basic & Clinical Science Poster
2001: Burden of Metabolic Bone Disease in Patients with IgG4-Related Disease with and Without Autoimmune Pancreatitis
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- GK
Guy Katz, MD
Massachusetts General Hospital
Boston, MA, United StatesDisclosure(s): No financial relationships with ineligible companies to disclose
Abstract Poster Presenter(s)
Guy Katz1, Aubree McMahon1, Grace McMahon1, Isha Jha1, Marcy Bolster2, Ana Fernandes1, Zachary Wallace3, Cory Perugino1, John Stone4 and Yasmin Hernandez-Barco1, 1Massachusetts General Hospital, Boston, MA, 2Massachusetts General Hospital, Concord, MA, 3Massachusetts General Hospital, Newton, MA, 4Massachusetts General Hospital Rheumatology Unit, Harvard Medical School, Concord, MA
Background/Purpose: Metabolic bone disease (MBD), including osteopenia and osteoporosis, is common in patients with inflammatory disorders, due to disease factors and glucocorticoid (GC) use, and in those with chronic pancreatitis, due to exocrine pancreatic insufficiency (EPI). IgG4-related disease (IgG4-RD) is a systemic immune-mediated disease that commonly causes autoimmune pancreatitis (AIP) and resultant EPI. We aimed to evaluate the screening frequency and prevalence of MBD in a large single-center cohort of patients with IgG4-RD, comparing those with and without AIP.
Methods: We retrospectively assessed for the presence of MBD and associated risk factors and treatments in all patients who met ACR/EULAR Classification Criteria for IgG4-RD in our cohort using two parallel study designs. First, we conducted a chart review and extracted details on risk factors, screening frequencies, prevalence, and treatment of MBD. We limited the chart review to patients who have close follow-up in our system, defined as 1) primary care provider (PCP) in the system with ≥ 1 visit with the PCP in the previous year; or 2) ≥ 2 years of follow-up, ≥ 3 total visits in our system in the last year, and ≥ 1 visit with a non-rheumatology provider in our system in the last year. Second, we sent surveys to all living subjects in our cohort with up-to-date contact information and collected patient-reported data on MBD prevalence, risk factors, screening, and treatment. Unpaired T tests and Chi-square tests were used to compare continuous and categorical variables, respectively, between patients with and without AIP.
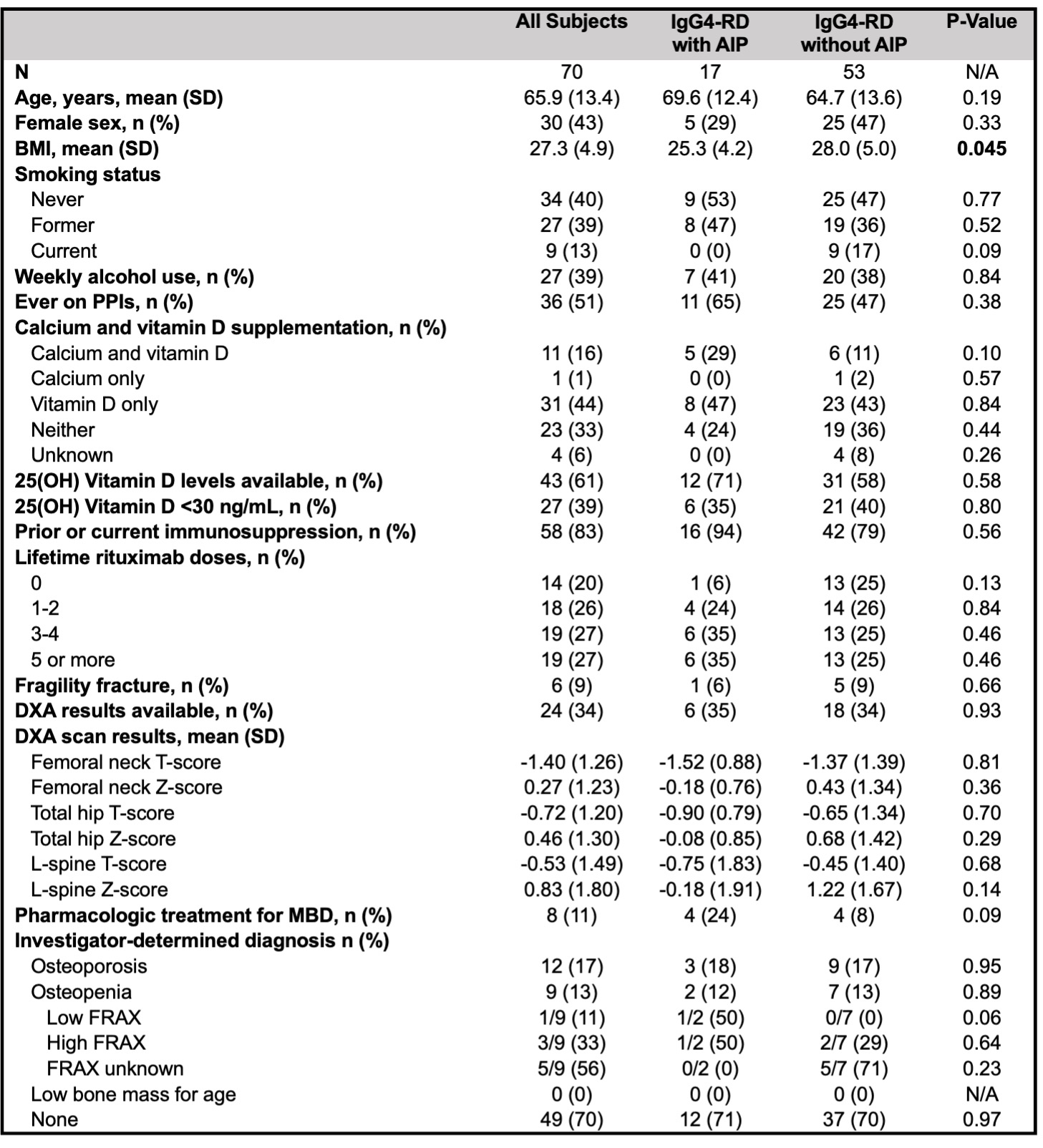
Results: 70 subjects (n=17 with AIP and n=53 without) met inclusion criteria for the chart review. Demographics and data on MBD, extracted from chart review, are summarized in Table 1. Subjects with AIP had a significantly lower mean BMI than those without. Otherwise, risk factors and MBD characteristics were similar between the two groups. Mean age was 66 years. Dual-energy X-ray absorptiometry (DXA) scans were performed in 24 (34%), and 21 subjects (30%) met World Health Organization criteria for osteoporosis or osteopenia.
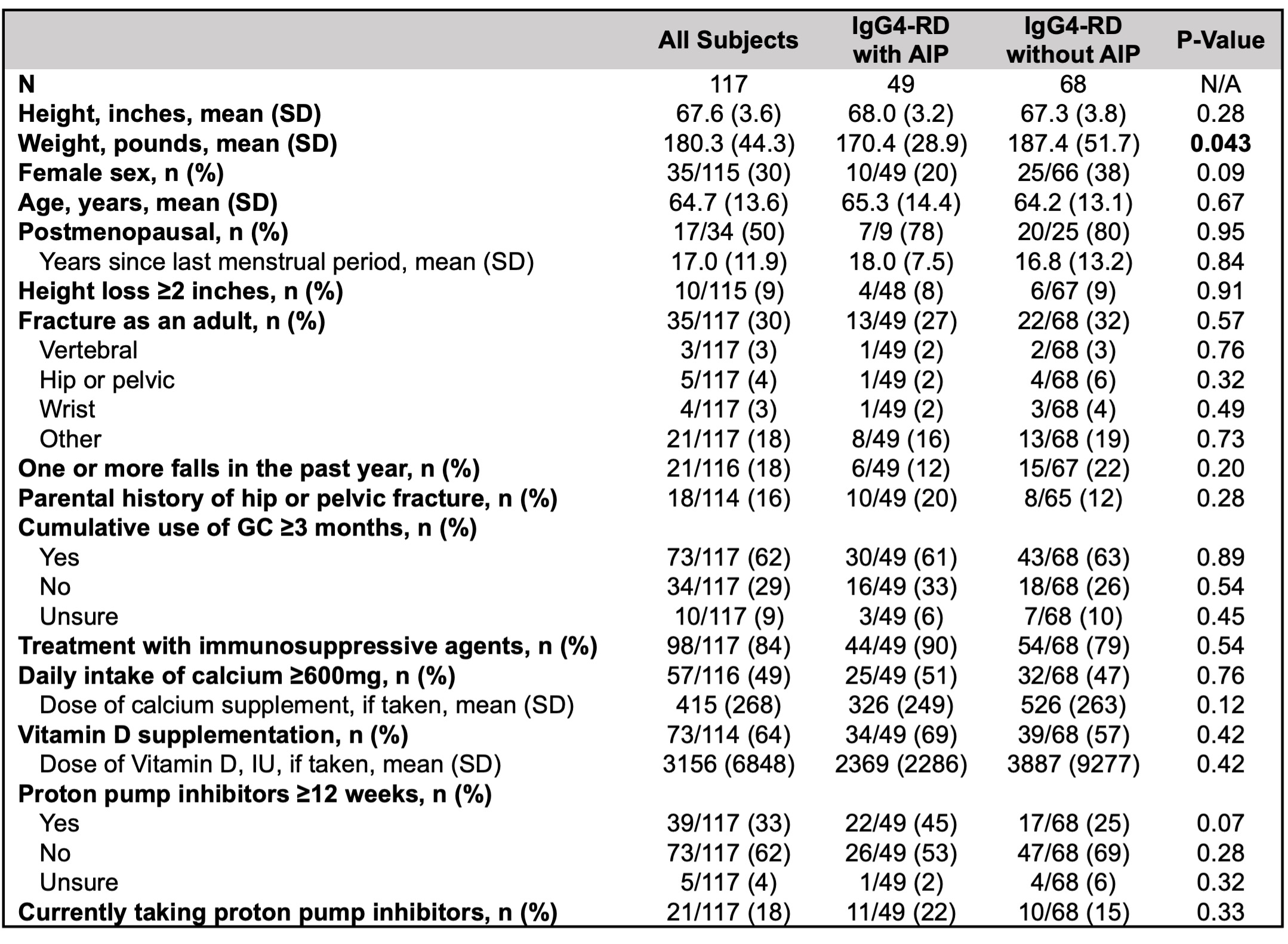
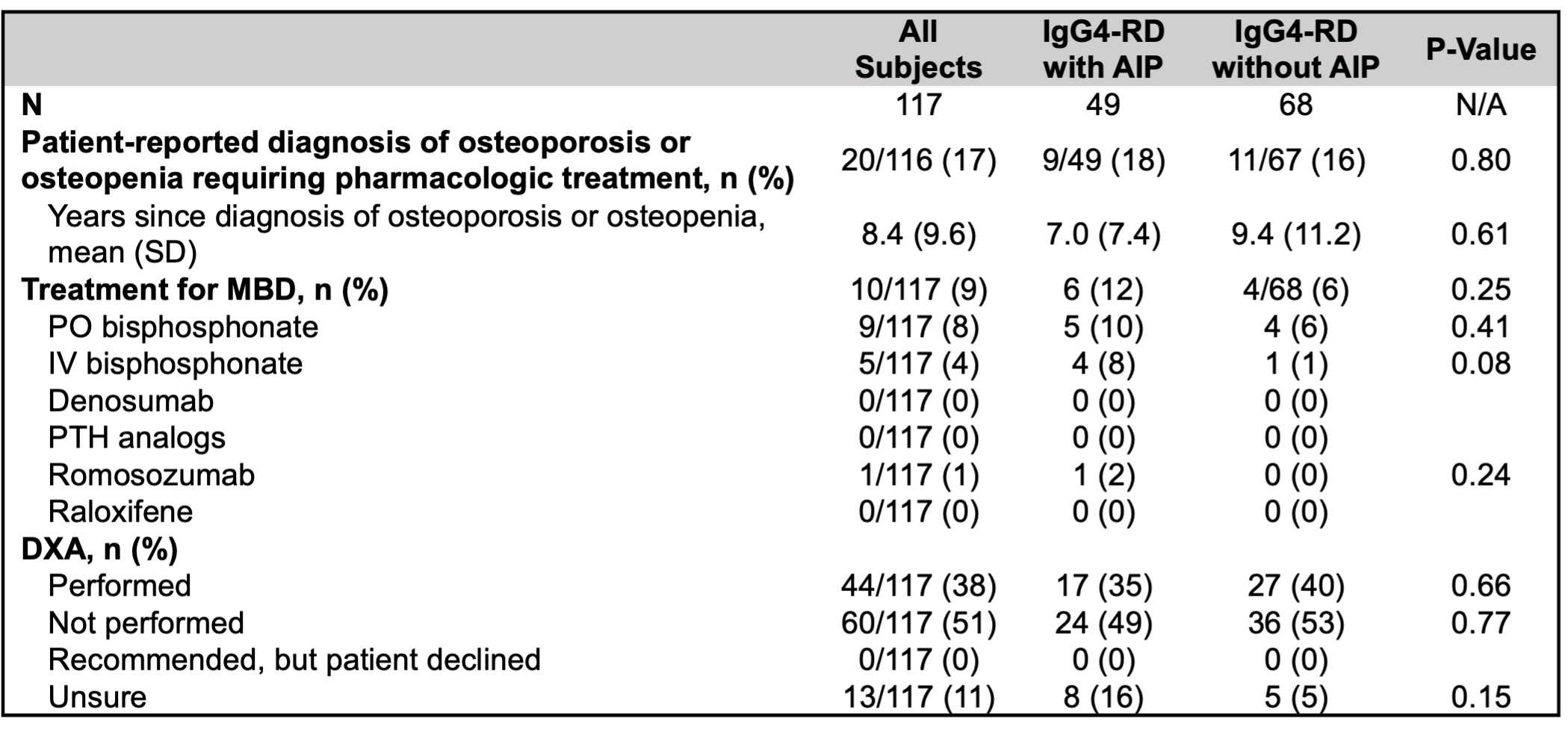
Of 278 patient surveys distributed, 117 (42%) were completed (49 with AIP and 68 without). In the patient-reported data, weight was significantly lower in subjects with AIP, but other demographics and MBD risk factors were similar (Table 2). 73 subjects (62%) reported ≥ 3 months of cumulative GC exposure, yet only 44 (38%) had a DXA recorded. Moreover, 20 subjects (17%) reported receiving a diagnosis of MBD requiring pharmacologic intervention, yet 10 (9%) reported taking medication for MBD.
Conclusion: MBD and associated risk factors are common in patients with IgG4-RD. Although our study was underpowered to detect between-group differences, patients with AIP had lower weight and BMI than those without, emphasizing that this population may be at higher risk for MBD due to EPI. Despite high rates of MBD risk factors, DXA screening frequency was low, and patient-reported prescriptions and adherence to pharmacologic treatment were low. These results highlight the need for clinicians to consider MBD when managing patients with IgG4-RD. Further studies are warranted to better define and address the burden of MBD in this population.
G. Katz: None; A. McMahon: None; G. McMahon: None; I. Jha: None; M. Bolster: American Board of Internal Medicine, 6, American College of Rheumatology, 4, Corbus, 5, Cumberland, 5, Elsevier, 2, Mitsubishi, 5, Novartis, 1, Rheumatology Research Foundation, 5, The Merck Manual, 6; A. Fernandes: None; Z. Wallace: BioCryst, 2, Bristol-Myers Squibb(BMS), 5, Horizon, 1, 2, 5, MedPace, 2, Novartis, 1, PPD, 2, Sanofi, 1, 5, Shionogi, 1, Visterra, 1, 2, Zenas, 1, 2; C. Perugino: Horizon Therapeutics, 2; J. Stone: Abvie, 2, Amgen, 1, 2, Argenx, 2, Aztrazeneca, 2, Bristol Myers Squibb, 2, 5, Celgene, 2, Chemocentryx, 2, Chugai, 2, GSK, 2, Horizon Therapeutics, 1, 2, 5, InflaRx, 2, IQVIA, 1, 2, Kyverna, 2, Mirabio, 2, NIH, 5, Novartis, 2, PPD, 2, Prometheus, 2, Q32, 2, Regeneron, 2, Roche-Genentech, 2, Roivant, 2, Sanofi, 2, 5, Spruce Biosciences, 2, Star Therapeutics, 2, Steritas, 12, Chair, Scientific Advisory Board (no fiduciary responsibilities), ZenasBio, 2; Y. Hernandez-Barco: Nestle Health Science, 1.
Background/Purpose: Metabolic bone disease (MBD), including osteopenia and osteoporosis, is common in patients with inflammatory disorders, due to disease factors and glucocorticoid (GC) use, and in those with chronic pancreatitis, due to exocrine pancreatic insufficiency (EPI). IgG4-related disease (IgG4-RD) is a systemic immune-mediated disease that commonly causes autoimmune pancreatitis (AIP) and resultant EPI. We aimed to evaluate the screening frequency and prevalence of MBD in a large single-center cohort of patients with IgG4-RD, comparing those with and without AIP.
Methods: We retrospectively assessed for the presence of MBD and associated risk factors and treatments in all patients who met ACR/EULAR Classification Criteria for IgG4-RD in our cohort using two parallel study designs. First, we conducted a chart review and extracted details on risk factors, screening frequencies, prevalence, and treatment of MBD. We limited the chart review to patients who have close follow-up in our system, defined as 1) primary care provider (PCP) in the system with ≥ 1 visit with the PCP in the previous year; or 2) ≥ 2 years of follow-up, ≥ 3 total visits in our system in the last year, and ≥ 1 visit with a non-rheumatology provider in our system in the last year. Second, we sent surveys to all living subjects in our cohort with up-to-date contact information and collected patient-reported data on MBD prevalence, risk factors, screening, and treatment. Unpaired T tests and Chi-square tests were used to compare continuous and categorical variables, respectively, between patients with and without AIP.
Results: 70 subjects (n=17 with AIP and n=53 without) met inclusion criteria for the chart review. Demographics and data on MBD, extracted from chart review, are summarized in Table 1. Subjects with AIP had a significantly lower mean BMI than those without. Otherwise, risk factors and MBD characteristics were similar between the two groups. Mean age was 66 years. Dual-energy X-ray absorptiometry (DXA) scans were performed in 24 (34%), and 21 subjects (30%) met World Health Organization criteria for osteoporosis or osteopenia.
Of 278 patient surveys distributed, 117 (42%) were completed (49 with AIP and 68 without). In the patient-reported data, weight was significantly lower in subjects with AIP, but other demographics and MBD risk factors were similar (Table 2). 73 subjects (62%) reported ≥ 3 months of cumulative GC exposure, yet only 44 (38%) had a DXA recorded. Moreover, 20 subjects (17%) reported receiving a diagnosis of MBD requiring pharmacologic intervention, yet 10 (9%) reported taking medication for MBD.
Conclusion: MBD and associated risk factors are common in patients with IgG4-RD. Although our study was underpowered to detect between-group differences, patients with AIP had lower weight and BMI than those without, emphasizing that this population may be at higher risk for MBD due to EPI. Despite high rates of MBD risk factors, DXA screening frequency was low, and patient-reported prescriptions and adherence to pharmacologic treatment were low. These results highlight the need for clinicians to consider MBD when managing patients with IgG4-RD. Further studies are warranted to better define and address the burden of MBD in this population.

Risk factors and characteristics of metabolic bone disease by chart review. BMI: body mass index, PPIs: proton pump inhibitors, AIP: autoimmune pancreatitis, DXA: dual-energy X-ray absorptiometry, MBD: metabolic bone disease.

Patient-reported demographics and metabolic bone disease risk factors in patients with IgG4-related disease with and without autoimmune pancreatitis. AIP: autoimmune pancreatitis, GC: glucocorticoids, IU: international units.

Patient-reported prevalence and treatment of metabolic bone disease in patients with IgG4-RD with and without autoimmune pancreatitis. AIP: autoimmune pancreatitis, MBD: metabolic bone disease, DXA: dual-energy X-ray absorptiometry.
G. Katz: None; A. McMahon: None; G. McMahon: None; I. Jha: None; M. Bolster: American Board of Internal Medicine, 6, American College of Rheumatology, 4, Corbus, 5, Cumberland, 5, Elsevier, 2, Mitsubishi, 5, Novartis, 1, Rheumatology Research Foundation, 5, The Merck Manual, 6; A. Fernandes: None; Z. Wallace: BioCryst, 2, Bristol-Myers Squibb(BMS), 5, Horizon, 1, 2, 5, MedPace, 2, Novartis, 1, PPD, 2, Sanofi, 1, 5, Shionogi, 1, Visterra, 1, 2, Zenas, 1, 2; C. Perugino: Horizon Therapeutics, 2; J. Stone: Abvie, 2, Amgen, 1, 2, Argenx, 2, Aztrazeneca, 2, Bristol Myers Squibb, 2, 5, Celgene, 2, Chemocentryx, 2, Chugai, 2, GSK, 2, Horizon Therapeutics, 1, 2, 5, InflaRx, 2, IQVIA, 1, 2, Kyverna, 2, Mirabio, 2, NIH, 5, Novartis, 2, PPD, 2, Prometheus, 2, Q32, 2, Regeneron, 2, Roche-Genentech, 2, Roivant, 2, Sanofi, 2, 5, Spruce Biosciences, 2, Star Therapeutics, 2, Steritas, 12, Chair, Scientific Advisory Board (no fiduciary responsibilities), ZenasBio, 2; Y. Hernandez-Barco: Nestle Health Science, 1.



