Poster Session B
Osteoarthritis (OA) and related disorders
Session: (1183–1199) Osteoarthritis – Clinical Poster II
1183: CoLchicine for Treatment of OsteoArthritis of the Knee—Updated Data from a Double-blind, Placebo-controlled Trial
Monday, November 13, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- JS
Jonathan Samuels, MD, RhMSUS
NYU Langone
Rye Brook, NY, United StatesDisclosure(s): No financial relationships with ineligible companies to disclose
Abstract Poster Presenter(s)
Katherine Tse1, Roni Meidan2, Michael Toprover3, David Wei2, Nicole Leung4, Maryfe Coronel2, Julia Cai2, Aryan Jain2, maria lessa2, Renata La Rocca Vieira2, Svetlana Krasnokutsky Samuels2, Michael Pillinger5 and Jonathan Samuels6, 1NYU Langone, New York, NY, 2NYU Langone Helath, New York, NY, 3New York University Langone Health, New York, NY, 4New York University Langone Hospital, New York, NY, 5New York University Grossman School of Medicine, New York, NY, 6NYU Langone, Rye Brook, NY
Background/Purpose: Knee osteoarthritis (OA) has a probable inflammatory role for IL-1b. The presence of calcium and urate crystals may contribute to OA by activating the NLRP3 inflammasome, leading to IL-1b production. Colchicine is a well-tolerated anti-inflammatory drug that inhibits the inflammasome and suppresses IL-1b. Prior studies investigating the efficacy of colchicine on knee OA include some reporting pain relief, others showing improvement in inflammatory markers, but none assessing synovial effusions.
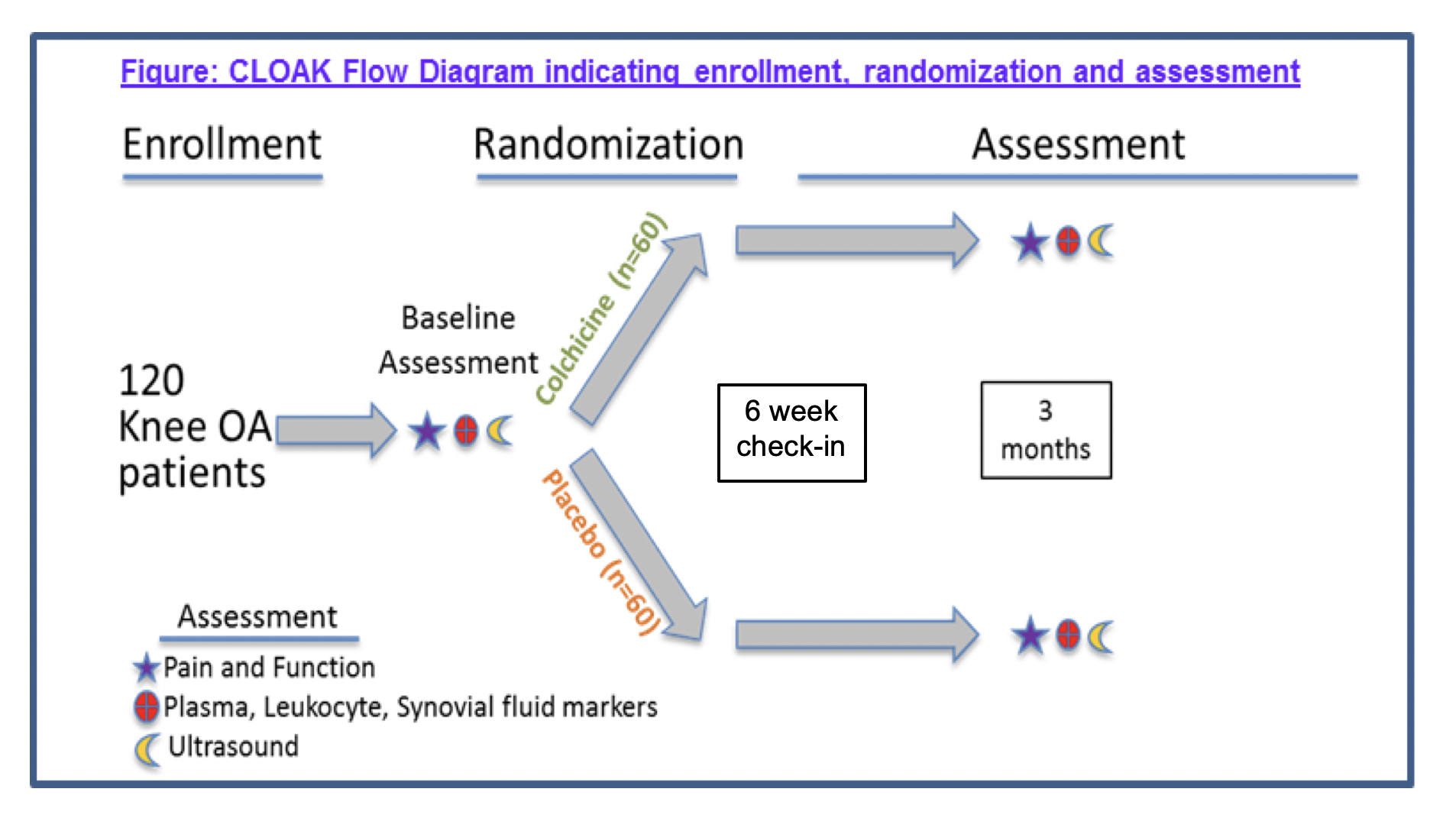
Methods: CLOAK is a randomized, double-blind, placebo-controlled trial of colchicine (0.6 mg daily for 3 months) for knee OA (Fig. 1). We are enrolling individuals ≥ 40 years of age, BMI ≤ 30 kg/m2, with symptomatic knee OA and Kellgren-Lawrence grade 2 or 3 on radiographs, who are willing to abstain from other anti-inflammatory treatment during the trial. Clinical outcomes include the change in knee pain by visual analog scale (VAS), and in Knee injury and Osteoarthritis Outcome Scores (KOOS) in the colchicine and placebo groups. If present, synovial fluid is aspirated and analyzed. Biologic outcomes to be assessed after study completion include changes in inflammatory markers in plasma, peripheral blood leukocytes, and synovial fluid.
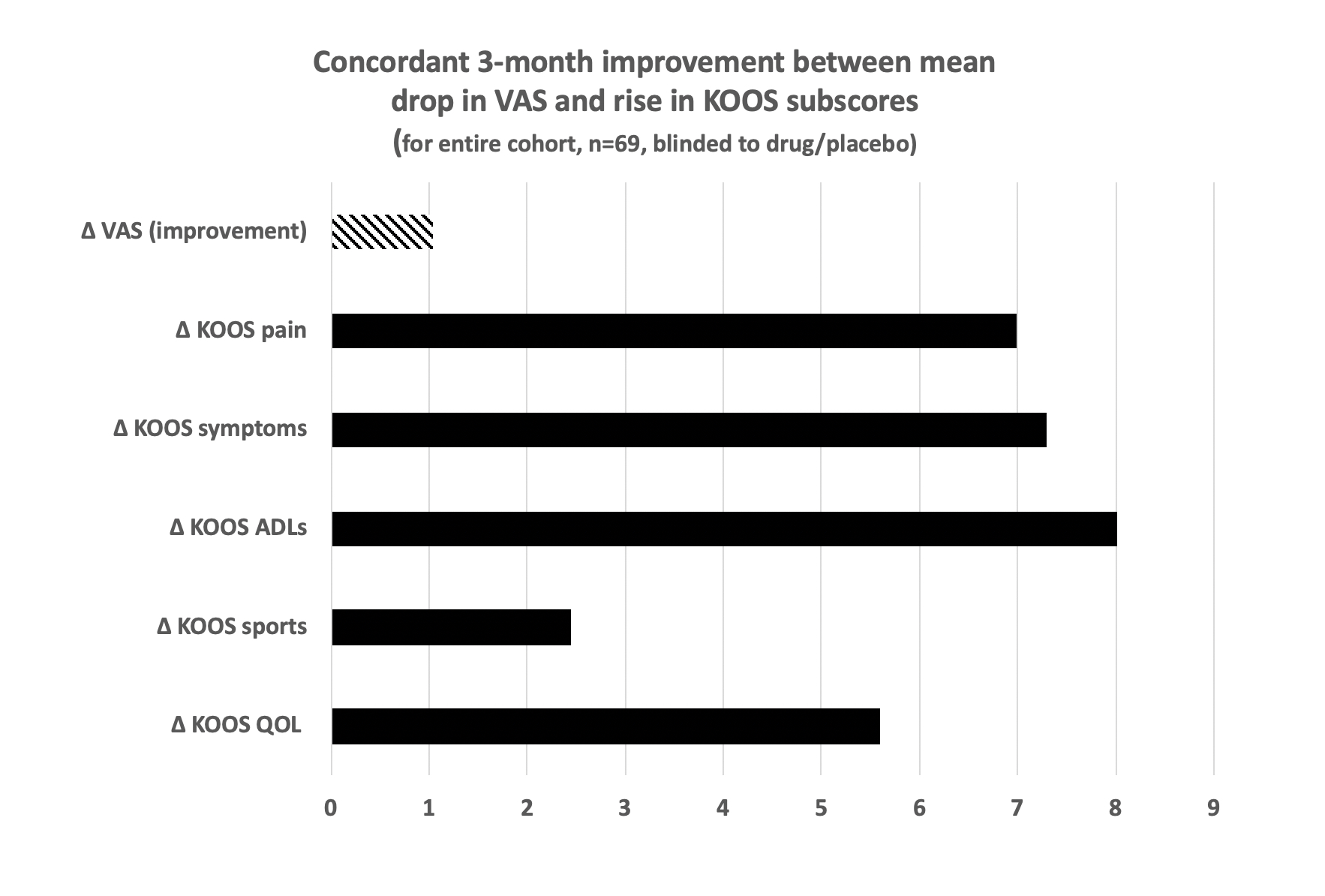
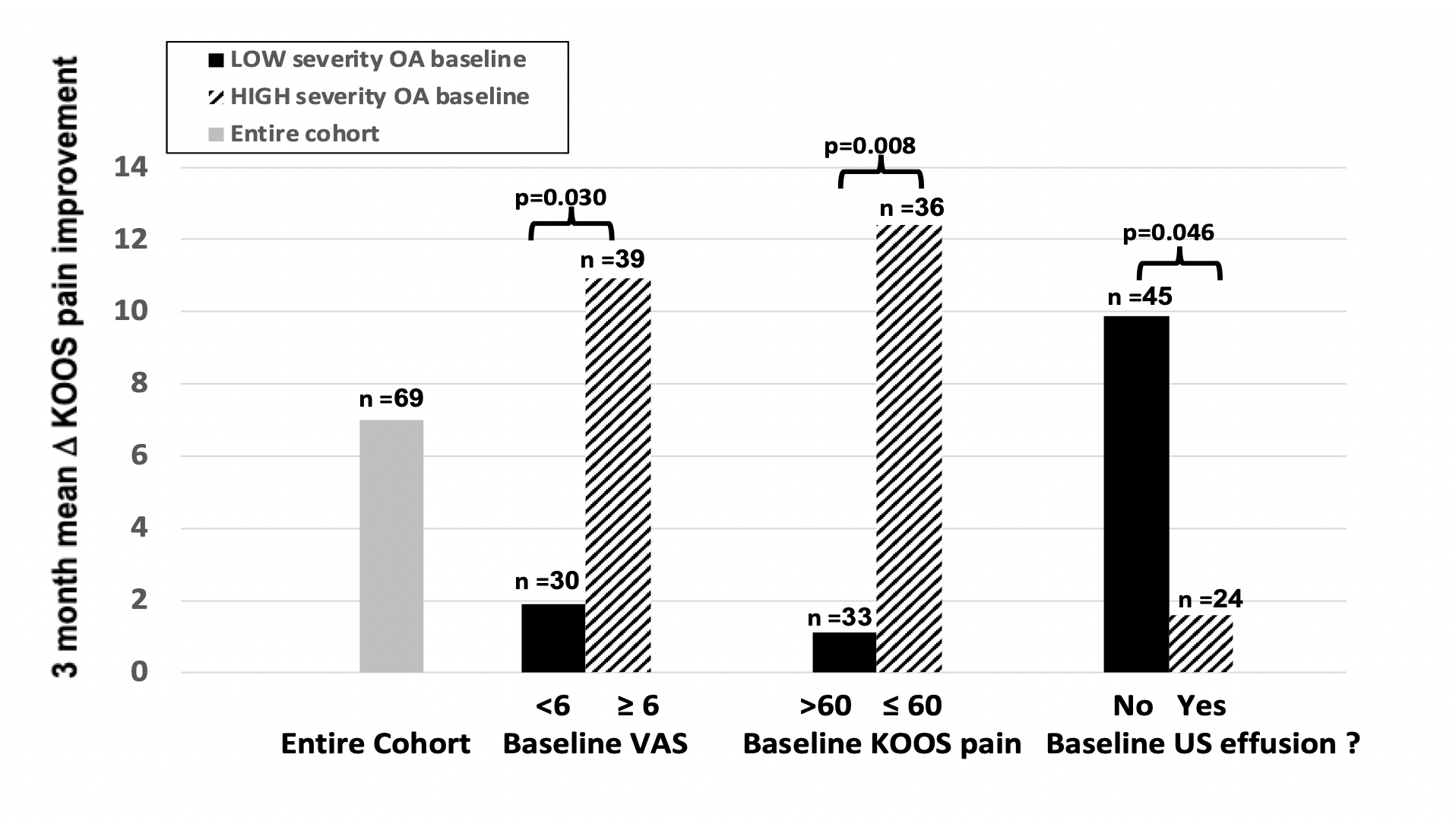
Results: To date, 1,105 potential subjects were contacted, 119 screened, and 97 enrolled. 69 have finished the study with data available: 32% male, 64% White, 24% Black, 4% Asian and 8% other, with mean±SD BMI 27.5±3.9 kg/m2 and age 68.6±10.1 years. Inclusive of all completing participants (blinded to treatment vs placebo) over the 3 months, mean VAS pain improved (decreased) by -1.04 units in the index knee; 58% demonstrated VAS improvement while 42% had no change or worsened. The mean VAS improvement was concordant with improvements (increases) in all 5 KOOS component subscores (Fig. 2), including mean pain (6.99), symptoms (7.30), activities of daily living (8.01), sports activity (2.45), and quality of life (5.59). Subsets of patients with baseline VAS ≥6 and baseline KOOS ≤60 for pain (i.e., more severe) showed significantly more 3-month mean KOOS pain improvement, even with the blinded inclusion of placebo, than those with less baseline severity (by baseline VAS: 10.8 vs. 1.95, p=0.030; by baseline KOOS 12.2 vs 1.1, p=0.008) (Fig. 3). Sonography was performed on all 69 subjects pre- and post-treatment. Subjects without effusion at baseline were more likely to experience knee pain improvement post treatment than those with effusion (mean KOOS improvement 9.8 vs 1.6, p=0.046).The prevalence of effusions did not differ pre and post treatment (29 vs 26%, ns).
Conclusion: The findings of this blinded analysis are consistent with the possibility that colchicine may offer benefit in terms of pain, function, and effusion in subjects with knee OA not taking other anti-inflammatory agents. Patients with higher baseline pain severity were more likely to improve, whereas patients with effusions at baseline were less likely to improve, with regard to post-treatment VAS and KOOS pain scores. Enrollment is ongoing, and the study will be unblinded and fully analyzed upon completion. Supported by an investigator-initiated grant from Hikma Pharmaceuticals.
K. Tse: None; R. Meidan: None; M. Toprover: ANI Pharmaceuticals, 2, Horizon Therapeutics, 5; D. Wei: None; N. Leung: None; M. Coronel: None; J. Cai: None; A. Jain: None; m. lessa: None; R. La Rocca Vieira: None; S. Krasnokutsky Samuels: None; M. Pillinger: Federation Bio, 2, Fortress Biotech, 2, Hikma, 5, Horizon Therapeutics, 2, 5, Scilex, 2, Sobi, 2; J. Samuels: None.
Background/Purpose: Knee osteoarthritis (OA) has a probable inflammatory role for IL-1b. The presence of calcium and urate crystals may contribute to OA by activating the NLRP3 inflammasome, leading to IL-1b production. Colchicine is a well-tolerated anti-inflammatory drug that inhibits the inflammasome and suppresses IL-1b. Prior studies investigating the efficacy of colchicine on knee OA include some reporting pain relief, others showing improvement in inflammatory markers, but none assessing synovial effusions.
Methods: CLOAK is a randomized, double-blind, placebo-controlled trial of colchicine (0.6 mg daily for 3 months) for knee OA (Fig. 1). We are enrolling individuals ≥ 40 years of age, BMI ≤ 30 kg/m2, with symptomatic knee OA and Kellgren-Lawrence grade 2 or 3 on radiographs, who are willing to abstain from other anti-inflammatory treatment during the trial. Clinical outcomes include the change in knee pain by visual analog scale (VAS), and in Knee injury and Osteoarthritis Outcome Scores (KOOS) in the colchicine and placebo groups. If present, synovial fluid is aspirated and analyzed. Biologic outcomes to be assessed after study completion include changes in inflammatory markers in plasma, peripheral blood leukocytes, and synovial fluid.
Results: To date, 1,105 potential subjects were contacted, 119 screened, and 97 enrolled. 69 have finished the study with data available: 32% male, 64% White, 24% Black, 4% Asian and 8% other, with mean±SD BMI 27.5±3.9 kg/m2 and age 68.6±10.1 years. Inclusive of all completing participants (blinded to treatment vs placebo) over the 3 months, mean VAS pain improved (decreased) by -1.04 units in the index knee; 58% demonstrated VAS improvement while 42% had no change or worsened. The mean VAS improvement was concordant with improvements (increases) in all 5 KOOS component subscores (Fig. 2), including mean pain (6.99), symptoms (7.30), activities of daily living (8.01), sports activity (2.45), and quality of life (5.59). Subsets of patients with baseline VAS ≥6 and baseline KOOS ≤60 for pain (i.e., more severe) showed significantly more 3-month mean KOOS pain improvement, even with the blinded inclusion of placebo, than those with less baseline severity (by baseline VAS: 10.8 vs. 1.95, p=0.030; by baseline KOOS 12.2 vs 1.1, p=0.008) (Fig. 3). Sonography was performed on all 69 subjects pre- and post-treatment. Subjects without effusion at baseline were more likely to experience knee pain improvement post treatment than those with effusion (mean KOOS improvement 9.8 vs 1.6, p=0.046).The prevalence of effusions did not differ pre and post treatment (29 vs 26%, ns).
Conclusion: The findings of this blinded analysis are consistent with the possibility that colchicine may offer benefit in terms of pain, function, and effusion in subjects with knee OA not taking other anti-inflammatory agents. Patients with higher baseline pain severity were more likely to improve, whereas patients with effusions at baseline were less likely to improve, with regard to post-treatment VAS and KOOS pain scores. Enrollment is ongoing, and the study will be unblinded and fully analyzed upon completion. Supported by an investigator-initiated grant from Hikma Pharmaceuticals.

Figure 1. Flow diagram of study plan.

Figure 2. Concordant 3-month improvement with mean drop in VAS and rise in KOOS subscores

Figure 3. Subject improvement in KOOS score from beginning to end of study, according to high or low baseline severity as measured by VAS and KOOS scores and presence of synovial effusion
K. Tse: None; R. Meidan: None; M. Toprover: ANI Pharmaceuticals, 2, Horizon Therapeutics, 5; D. Wei: None; N. Leung: None; M. Coronel: None; J. Cai: None; A. Jain: None; m. lessa: None; R. La Rocca Vieira: None; S. Krasnokutsky Samuels: None; M. Pillinger: Federation Bio, 2, Fortress Biotech, 2, Hikma, 5, Horizon Therapeutics, 2, 5, Scilex, 2, Sobi, 2; J. Samuels: None.



