Poster Session C
Systemic lupus erythematosus (SLE)
Session: (2326–2351) SLE – Treatment Poster III
2330: Disease-Related Outcomes of Cognitive Behavioral Therapy in Randomized Control Trial for Youth with Childhood-onset SLE: A Secondary Analysis
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall

Andrea Knight, MD, MSCE
Hospital for Sick Children
Toronto, ON, CanadaDisclosure(s): Pfizer: Speaker/Honoraria (includes speakers bureau, symposia, and expert witness) (Ongoing)
Abstract Poster Presenter(s)
Natoshia Cunningham1, Michelle Adler1, Ashley Danguecan2, Mallet Reid1, Samantha Ely3, Mathew Reeves4, Lawrence Ng2, Paris Moaf2, Tala El Tal5, Sarah Mossad2, Luana Flores Pereira2, Deborah Levy2, Linda Hiraki2, Jennifer Stinson2, Sara Ahola Kohut2, khalid abulaban6, Elizabeth Kessler7, Stacy Allen8, Tamar Rubinstein9, Evin Rothschild10, Natalie Rosenwasser11, Kabita Nanda12, Susan Canny13, Emily Smitherman14, Livie Huie15, James Birmingham16, Ekemini Ogbu17, Hermine Brunner18, Dhriti Sharma19, Allison Thompson20, Janel Thompson21, Miranda Moyer20, Emily Nguyen20, Angela Chapson20 and Andrea Knight2, 1Michigan State University, Grand Rapids, MI, 2The Hospital for Sick Children, Toronto, ON, Canada, 3Michigan State University, Wayne State University, Grand Rapids, MI, 4Michigan State University, East Lansing, MI, 5Children's Hospital of Eastern Ontario (CHEO), Ottawa, ON, Canada, 6Helen DeVos Children's Hospital, Ada, MI, 7Spectrum Health, Grand Rapids, MI, 8Helen DeVos Children's Hospital, Caledonia, MI, 9Albert Einstein College of Medicine, Children's Hospital at Montefiore, White Plains, NY, 10Albert Einstein Medical Center, Children's Hospital at Montefiore, Bronx, NY, 11Seattle Children's Hospital, Seattle, WA, 12Seattle Children's Hospital/University of Washington, Seattle, WA, 13Seattle Children's Hospital/University of Washington, Seattle, WA, 14University of Alabama at Birmingham, Birmingham, AL, 15University of Alabama at Birmingham, Gardendale, AL, 16Self, Ada, MI, 17Cincinnati Children's Hospital Medical Center, University of Cincinnati School of Medicine, Johns Hopkins University, Cincinnati, OH, 18Cincinnati Children's Hospital Medical Center, Division of Rheumatology, Cincinnati, OH, 19Cincinnati Children's Hospital Medical Center, Cincinnati, OH, 20Patient Co-Investigative Team, n/a, 21Patient Co-Investigative Team, Revere, PA
Background/Purpose: Childhood-onset systemic lupus erythematosus (cSLE) is associated with symptoms such as fatigue, pain, and depressive symptoms that contribute to poor health-related quality of life. The Treatment and Education Approach for Childhood-Onset Lupus (TEACH; a cognitive behavioral therapy (CBT) program) has been shown in our prior work to significantly reduce fatigue and depressive symptoms when compared with standard care. The current study is a secondary data analysis of this recently completed trial which explores disease-related outcomes following TEACH, including disease activity, medication adherence, and health-related quality of life.
Methods: We conducted a randomized controlled trial of 59 youth ages 12-22 years meeting the ACR diagnostic classification criteria for SLE, from six rheumatology sites across the U.S. and Canada. Eligible participants had elevations in at least one target symptom area (e.g., fatigue, pain, or depression). Participants completed a baseline assessment, were randomized to 1) TEACH, a remotely delivered six-week CBT program with medical treatment as usual (TAU), or 2) TAU alone, then completed a post-assessment 8 weeks later. The current study explored: i) physician-reported disease activity via the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) and a Visual Analog Scale (VAS, range 0-100); ii) medication adherence measured by the Medication Adherence Self-Report Inventory (MASRI, range 0-100%); and iii) health-related quality of life measured by Pediatrics Quality of Life – Adolescent version (PedsQL-A, 0-100). Independent samples t-tests compared changes from baseline to post-assessment between those who received TEACH+TAU vs. those who received TAU alone.
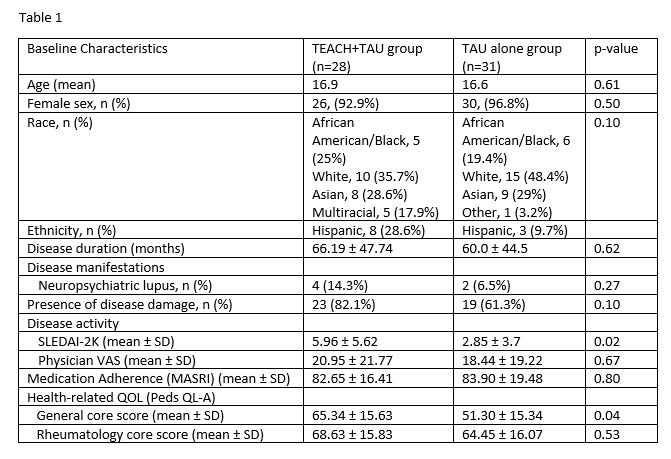
Results: Twenty-eight (47.5%) participants received TEACH+TAU, and 31 (52.5%) received TAU alone. Participant characteristics are shown in Table 1. At baseline, disease activity (SLEDAI-2K) and health-related quality of life (general core score) were higher in the TEACH+TAU group. The change in disease activity (SLEDAI-2K) scores between groups significantly differed, with the TEACH+TAU group demonstrating a significant decrease in disease activity scores (t(40) = 2.47, p = 0.02; Table 2, Figure 3A). There was also a statistically significant decrease in physician-reported disease activity VAS for the TEACH+TAU group vs the TAU group, (t(38) = 2.24, p = 0.031; Figure 3B). There were no other statistically significant differences between groups for changes in medication adherence or health-related quality of life.
Conclusion: TEACH may be associated with short-term improvement in disease activity, in addition to reducing depressive symptoms and fatigue. TEACH represents a promising treatment modality to mitigate mental health symptoms and improve disease outcomes. Future research will examine the long-term effectiveness of TEACH when implemented into real-world pediatric rheumatology clinics.
.jpg)
.jpg)
N. Cunningham: DoD, 12, DoD Transformative Vision Award (starting Oct 2023) and CARRA Transdisciplinary Research Grant (NCE). I also have other current funding support.; M. Adler: None; A. Danguecan: None; M. Reid: None; S. Ely: None; M. Reeves: None; L. Ng: None; P. Moaf: None; T. El Tal: None; S. Mossad: None; L. Flores Pereira: None; D. Levy: None; L. Hiraki: None; J. Stinson: None; S. Ahola Kohut: None; k. abulaban: None; E. Kessler: None; S. Allen: None; T. Rubinstein: None; E. Rothschild: None; N. Rosenwasser: None; K. Nanda: None; S. Canny: None; E. Smitherman: None; L. Huie: None; J. Birmingham: None; E. Ogbu: None; H. Brunner: AbbVie, 2, AstraZeneca-Medimmune, 2, Biogen, 2, Boehringer-Ingelheim, 2, Bristol-Myers Squibb (BMS), 2, 5, Celgene, 2, Eli Lilly, 2, 5, EMD Serono, 2, F-Hoffman La Roche, 2, 5, GlaxoSmithKlein (GSK), 2, 5, 6, Horizon, 2, 2, Janssen, 5, Merck, 2, Novartis, 2, 5, 6, Pfizer, 2, 5, 6; D. Sharma: None; A. Thompson: None; J. Thompson: None; M. Moyer: None; E. Nguyen: None; A. Chapson: None; A. Knight: Pfizer, 6.
Background/Purpose: Childhood-onset systemic lupus erythematosus (cSLE) is associated with symptoms such as fatigue, pain, and depressive symptoms that contribute to poor health-related quality of life. The Treatment and Education Approach for Childhood-Onset Lupus (TEACH; a cognitive behavioral therapy (CBT) program) has been shown in our prior work to significantly reduce fatigue and depressive symptoms when compared with standard care. The current study is a secondary data analysis of this recently completed trial which explores disease-related outcomes following TEACH, including disease activity, medication adherence, and health-related quality of life.
Methods: We conducted a randomized controlled trial of 59 youth ages 12-22 years meeting the ACR diagnostic classification criteria for SLE, from six rheumatology sites across the U.S. and Canada. Eligible participants had elevations in at least one target symptom area (e.g., fatigue, pain, or depression). Participants completed a baseline assessment, were randomized to 1) TEACH, a remotely delivered six-week CBT program with medical treatment as usual (TAU), or 2) TAU alone, then completed a post-assessment 8 weeks later. The current study explored: i) physician-reported disease activity via the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) and a Visual Analog Scale (VAS, range 0-100); ii) medication adherence measured by the Medication Adherence Self-Report Inventory (MASRI, range 0-100%); and iii) health-related quality of life measured by Pediatrics Quality of Life – Adolescent version (PedsQL-A, 0-100). Independent samples t-tests compared changes from baseline to post-assessment between those who received TEACH+TAU vs. those who received TAU alone.
Results: Twenty-eight (47.5%) participants received TEACH+TAU, and 31 (52.5%) received TAU alone. Participant characteristics are shown in Table 1. At baseline, disease activity (SLEDAI-2K) and health-related quality of life (general core score) were higher in the TEACH+TAU group. The change in disease activity (SLEDAI-2K) scores between groups significantly differed, with the TEACH+TAU group demonstrating a significant decrease in disease activity scores (t(40) = 2.47, p = 0.02; Table 2, Figure 3A). There was also a statistically significant decrease in physician-reported disease activity VAS for the TEACH+TAU group vs the TAU group, (t(38) = 2.24, p = 0.031; Figure 3B). There were no other statistically significant differences between groups for changes in medication adherence or health-related quality of life.
Conclusion: TEACH may be associated with short-term improvement in disease activity, in addition to reducing depressive symptoms and fatigue. TEACH represents a promising treatment modality to mitigate mental health symptoms and improve disease outcomes. Future research will examine the long-term effectiveness of TEACH when implemented into real-world pediatric rheumatology clinics.

Baseline characteristics of the TEACH+TAU group and TAU alone group.
.jpg)
Between group pre-post differences of disease-related outcomes.
.jpg)
Changes in SLEDAI-2K and Disease Activity VAS among TEACH and TAU participants from pre to post assessment.
N. Cunningham: DoD, 12, DoD Transformative Vision Award (starting Oct 2023) and CARRA Transdisciplinary Research Grant (NCE). I also have other current funding support.; M. Adler: None; A. Danguecan: None; M. Reid: None; S. Ely: None; M. Reeves: None; L. Ng: None; P. Moaf: None; T. El Tal: None; S. Mossad: None; L. Flores Pereira: None; D. Levy: None; L. Hiraki: None; J. Stinson: None; S. Ahola Kohut: None; k. abulaban: None; E. Kessler: None; S. Allen: None; T. Rubinstein: None; E. Rothschild: None; N. Rosenwasser: None; K. Nanda: None; S. Canny: None; E. Smitherman: None; L. Huie: None; J. Birmingham: None; E. Ogbu: None; H. Brunner: AbbVie, 2, AstraZeneca-Medimmune, 2, Biogen, 2, Boehringer-Ingelheim, 2, Bristol-Myers Squibb (BMS), 2, 5, Celgene, 2, Eli Lilly, 2, 5, EMD Serono, 2, F-Hoffman La Roche, 2, 5, GlaxoSmithKlein (GSK), 2, 5, 6, Horizon, 2, 2, Janssen, 5, Merck, 2, Novartis, 2, 5, 6, Pfizer, 2, 5, 6; D. Sharma: None; A. Thompson: None; J. Thompson: None; M. Moyer: None; E. Nguyen: None; A. Chapson: None; A. Knight: Pfizer, 6.



