Poster Session B
Vasculitis
Session: (1534–1553) Vasculitis – ANCA-Associated Poster II: Epidemiology, Outcomes, & Classification
1551: Measurement of Sinonasal Disease Activity in Granulomatosis with Polyangiitis
Monday, November 13, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- RR
Rennie Rhee, MD, MS
University of Pennsylvania
Philadelphia, PA, United StatesDisclosure(s): No financial relationships with ineligible companies to disclose
Abstract Poster Presenter(s)
Roger Yang1, Ellen Romich2, Shubhasree Banerjee3, Naomi Amudala3, Peter Merkel3, Joshua Baker3 and Rennie Rhee3, 1University of Pennsylvania, Montréal, QC, Canada, 2Hospital of the University of Pennsylvania, Media, PA, 3University of Pennsylvania, Philadelphia, PA
Background/Purpose: In granulomatosis with polyangiitis (GPA), sinonasal inflammation can be severe and significantly impact quality of life. Little is known about the most effective local and systemic therapies for sinonasal disease in GPA, in part due to outcome measures which lack sensitivity to change. The Sino-Nasal Outcome Test-22 (SNOT22) is a patient-reported outcome measure validated in chronic rhinosinusitis but it has not been well-studied in GPA. This study measured sinonasal disease activity using the SNOT22 and the more widely-used Birmingham Vasculitis Activity Score for Wegener's Granulomatosis (BVAS/WG) in patients treated for active GPA.
Methods: This prospective, longitudinal cohort study included patients who met the 1990 ACR classification criteria for GPA. Patients with active disease during enrollment and at least one follow-up visit 3 or more months later were included. The following therapy initiations were examined: I) saline nasal rinses; II) topical nasal glucocorticoids; III) topical nasal antibiotics; and IV) rituximab (within the prior 6 months); patients could be in more than one group. Outcome measures included: (a) percent of patients achieving the minimal clinically important difference (MCID) for SNOT22 of 8 as determined for chronic rhinosinusitis; (b) percent change in SNOT22; and (c) percent of patients achieving BVAS/WG=0 for sinonasal items. For analysis of MCID, regression models adjusted for baseline SNOT22 score and predicted values were plotted. In secondary analyses models also adjusted for potential confounders, including prednisone, other concurrent treatments, and active sinus disease at relapse visit.
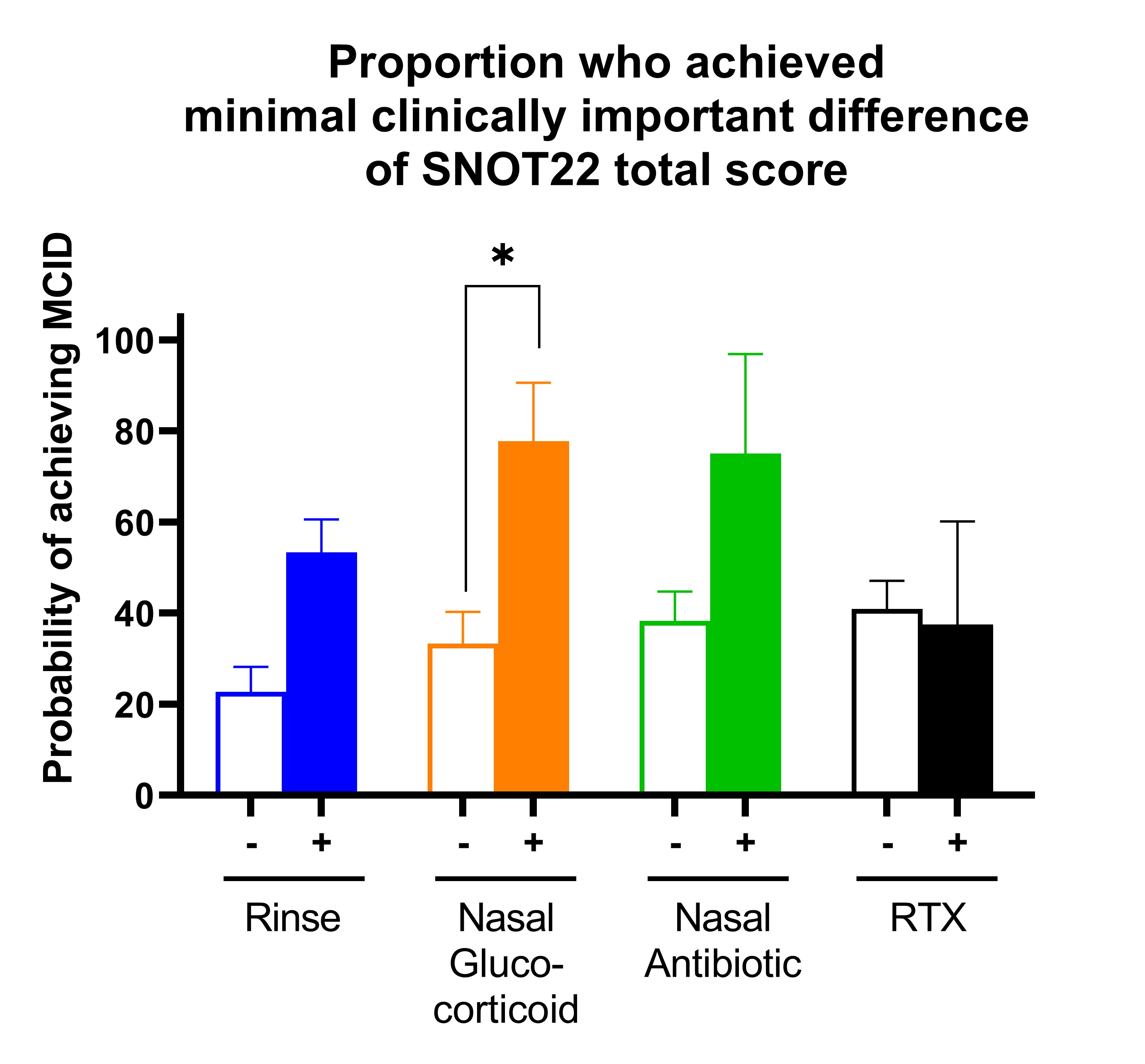
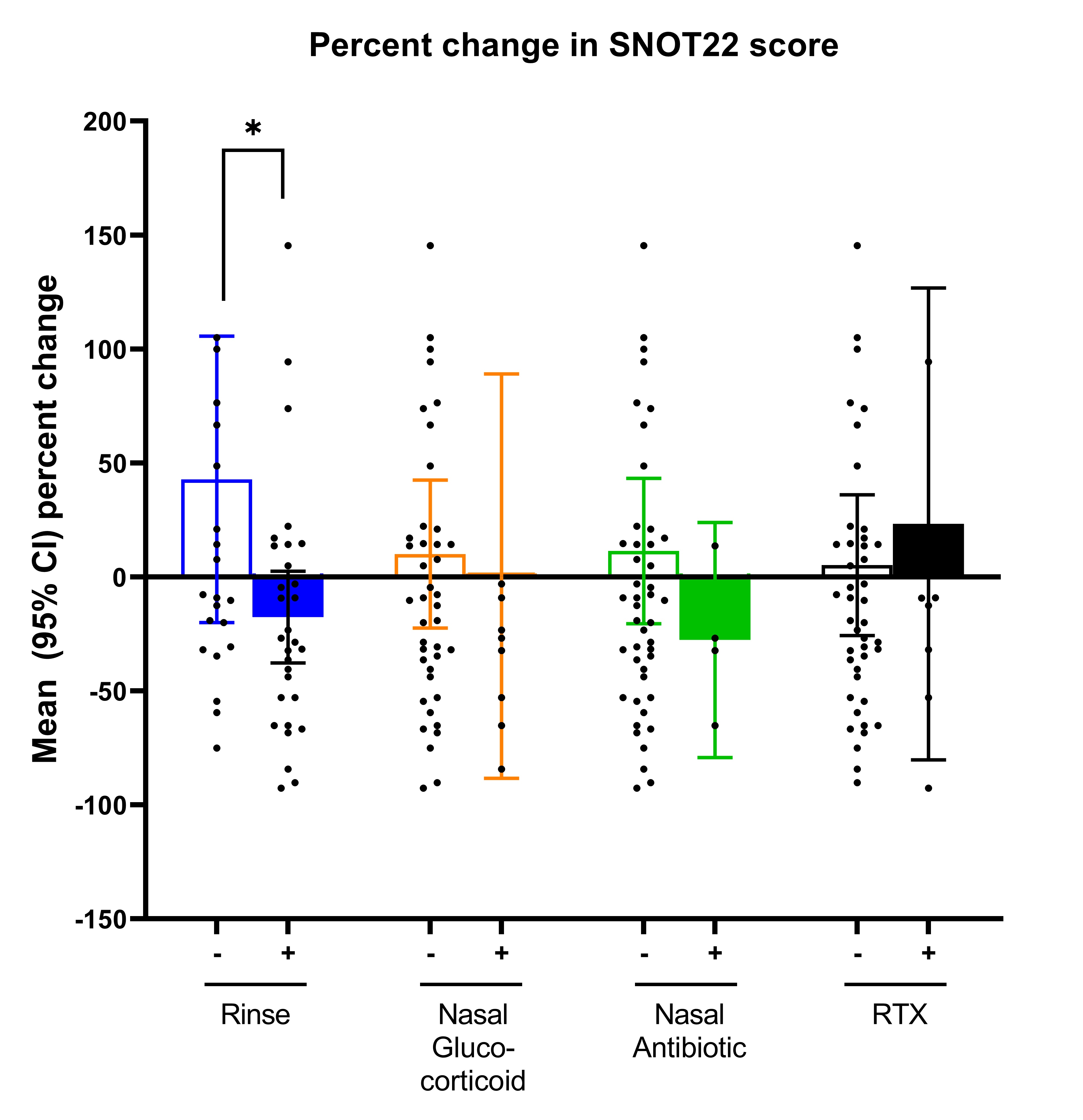
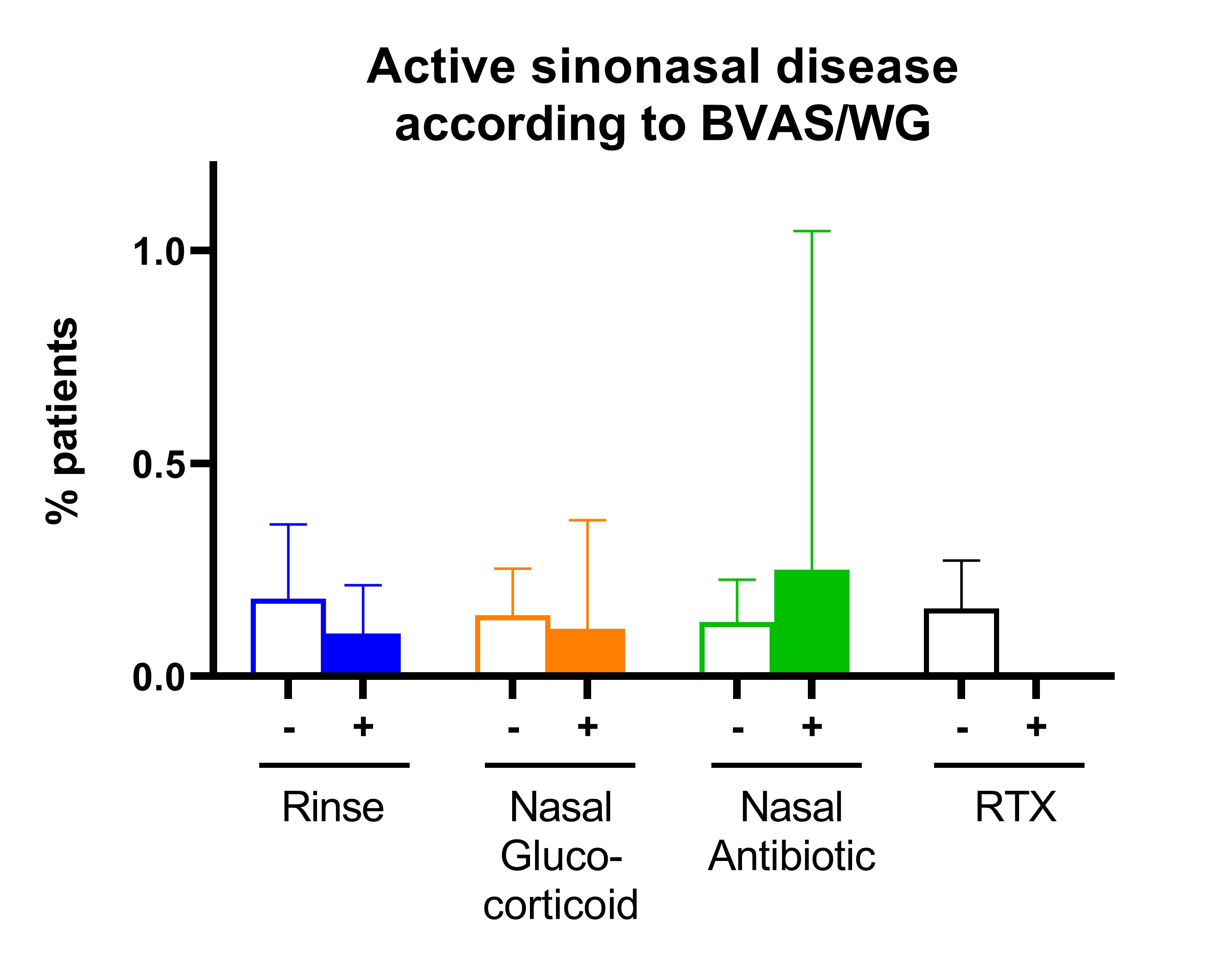
Results: The study included 52 flares in 42 patients with GPA. 35 (83%) patients had a prior history of sinonasal involvement, 28 (67%) were PR3-ANCA positive, and mean disease duration was 2 (0-5) years. At the active visit, 37 (70%) visits had active sinonasal involvement, and mean SNOT-22 score was 27 (IQR 4,15). New medications prescribed included: 30 (58%) saline nasal rinse, 9 (18%) topical nasal glucocorticoids, 4 (8%) topical nasal antibiotics, and 8 (15%) rituximab. For topical nasal glucocorticoids, new initiation vs. no initiation was associated with a greater probability of achieving an MCID for SNOT22 (OR 8.5 [95% CI 1.3, 55.6], P-value 0.026) (Figure 1). For saline nasal rinse, new initiation vs no initiation was associated with a greater percent change in SNOT22 (coefficient -0.60, [95% CI -1.17, -0.04], P= 0.037) (Figure 2). Results remain statistically significant even after adjusting for confounders. No significant changes were seen between any treatment groups when using the sinonasal items on BVAS/WG (Figure 3).
Conclusion: In GPA use of a patient-reported outcome measure of sinonasal symptoms (SNOT22) enabled detection of response to topical nasal therapies. Specifically, use of saline nasal rinse and topical nasal glucocorticoids were associated with improvement in sinonasal symptoms. In contrast, treatment response was not detected when using the BVAS/WG, suggesting that SNOT22 may have more responsiveness and convergent validity when assessing sinonasal disease in GPA.
R. Yang: None; E. Romich: None; S. Banerjee: None; N. Amudala: None; P. Merkel: AbbVie/Abbott, 5, Amgen, 2, 5, ArGenx, 2, AstraZeneca, 2, 5, Boehringer-Ingelheim, 2, 5, Bristol-Myers Squibb(BMS), 2, 5, Cabaletta, 2, CSL Behring, 2, Eicos, 5, Electra, 5, Genentech, 5, GlaxoSmithKlein(GSK), 2, 5, HiBio, 2, InflaRx, 2, 5, Janssen, 2, Jubilant, 2, Kyverna, 2, 11, MiroBio, 2, Neutrolis, 5, Novartis, 2, NS Pharma, 2, Q32, 2, Regeneron, 2, Sanofi, 2, Sparrow, 2, Takeda, 2, 5, UpToDate, 9, Visterra, 2; J. Baker: CorEvitas, 2, Cumberland Pharma, 2, Horizon Pharmaceuticals, 5; R. Rhee: None.
Background/Purpose: In granulomatosis with polyangiitis (GPA), sinonasal inflammation can be severe and significantly impact quality of life. Little is known about the most effective local and systemic therapies for sinonasal disease in GPA, in part due to outcome measures which lack sensitivity to change. The Sino-Nasal Outcome Test-22 (SNOT22) is a patient-reported outcome measure validated in chronic rhinosinusitis but it has not been well-studied in GPA. This study measured sinonasal disease activity using the SNOT22 and the more widely-used Birmingham Vasculitis Activity Score for Wegener's Granulomatosis (BVAS/WG) in patients treated for active GPA.
Methods: This prospective, longitudinal cohort study included patients who met the 1990 ACR classification criteria for GPA. Patients with active disease during enrollment and at least one follow-up visit 3 or more months later were included. The following therapy initiations were examined: I) saline nasal rinses; II) topical nasal glucocorticoids; III) topical nasal antibiotics; and IV) rituximab (within the prior 6 months); patients could be in more than one group. Outcome measures included: (a) percent of patients achieving the minimal clinically important difference (MCID) for SNOT22 of 8 as determined for chronic rhinosinusitis; (b) percent change in SNOT22; and (c) percent of patients achieving BVAS/WG=0 for sinonasal items. For analysis of MCID, regression models adjusted for baseline SNOT22 score and predicted values were plotted. In secondary analyses models also adjusted for potential confounders, including prednisone, other concurrent treatments, and active sinus disease at relapse visit.
Results: The study included 52 flares in 42 patients with GPA. 35 (83%) patients had a prior history of sinonasal involvement, 28 (67%) were PR3-ANCA positive, and mean disease duration was 2 (0-5) years. At the active visit, 37 (70%) visits had active sinonasal involvement, and mean SNOT-22 score was 27 (IQR 4,15). New medications prescribed included: 30 (58%) saline nasal rinse, 9 (18%) topical nasal glucocorticoids, 4 (8%) topical nasal antibiotics, and 8 (15%) rituximab. For topical nasal glucocorticoids, new initiation vs. no initiation was associated with a greater probability of achieving an MCID for SNOT22 (OR 8.5 [95% CI 1.3, 55.6], P-value 0.026) (Figure 1). For saline nasal rinse, new initiation vs no initiation was associated with a greater percent change in SNOT22 (coefficient -0.60, [95% CI -1.17, -0.04], P= 0.037) (Figure 2). Results remain statistically significant even after adjusting for confounders. No significant changes were seen between any treatment groups when using the sinonasal items on BVAS/WG (Figure 3).
Conclusion: In GPA use of a patient-reported outcome measure of sinonasal symptoms (SNOT22) enabled detection of response to topical nasal therapies. Specifically, use of saline nasal rinse and topical nasal glucocorticoids were associated with improvement in sinonasal symptoms. In contrast, treatment response was not detected when using the BVAS/WG, suggesting that SNOT22 may have more responsiveness and convergent validity when assessing sinonasal disease in GPA.

Figure 1: Proportion of patients with active GPA who achieved MCID of SNOT22 after new initiation of treatment.
Bar graphs show mean (95% CI) of probability of achieving the minimal clinically important difference (MCID) of the Sino-Nasal Outcome Test 22 (SNOT22) score. Four treatments were analyzed separately so each patient contributed to each analysis and were categorized based on no initiation of treatment (-) vs initiation of treatment (+). Four medications were evaluated: saline nasal rinse (blue), topical nasal glucocorticoids (orange), topical nasal antibiotic (green), and rituximab within the prior 6 months (RTX; black). Logistic regression models adjusted for baseline SNOT22 score at time of active disease. * P < 0.05.
Bar graphs show mean (95% CI) of probability of achieving the minimal clinically important difference (MCID) of the Sino-Nasal Outcome Test 22 (SNOT22) score. Four treatments were analyzed separately so each patient contributed to each analysis and were categorized based on no initiation of treatment (-) vs initiation of treatment (+). Four medications were evaluated: saline nasal rinse (blue), topical nasal glucocorticoids (orange), topical nasal antibiotic (green), and rituximab within the prior 6 months (RTX; black). Logistic regression models adjusted for baseline SNOT22 score at time of active disease. * P < 0.05.

Figure 2: Percent change in SNOT22 score after initiation of new treatment for active granulomatosis with polyangiitis.
Bar graphs show mean (95% CI) percent change in Sino-Nasal Outcome Test 22 (SNOT22) score between active and follow-up visits. Four different treatments were analyzed separately so each patient contributed to each analysis and were categorized based on no initiation of treatment (-) vs initiation of treatment (+). Four medications were evaluated: saline nasal rinse (blue), topical nasal glucocorticoids (orange), topical nasal antibiotic (green), and rituximab within past 6 months (RTX; black). * P < 0.05.
Bar graphs show mean (95% CI) percent change in Sino-Nasal Outcome Test 22 (SNOT22) score between active and follow-up visits. Four different treatments were analyzed separately so each patient contributed to each analysis and were categorized based on no initiation of treatment (-) vs initiation of treatment (+). Four medications were evaluated: saline nasal rinse (blue), topical nasal glucocorticoids (orange), topical nasal antibiotic (green), and rituximab within past 6 months (RTX; black). * P < 0.05.

Figure 3: Proportion of patients with sinonasal disease activity according to BVAS/WG after initiation of new treatment for active granulomatosis with polyangiitis.
Bar graphs show percentage (95% CI) of patients who had new/worsening or persistent sinonasal activity according to the BVAS/WG. Four different treatments were analyzed separately so each patient contributed to each analysis and were categorized based on no initiation of treatment (-) vs initiation of treatment (+).. Four medications were evaluated: saline nasal rinse (blue), topical nasal glucocorticoids (orange), topical nasal antibiotic (green), and rituximab within the prior 6 months (RTX; black). No statistically significant differences were found among the four treatment comparisons.
Bar graphs show percentage (95% CI) of patients who had new/worsening or persistent sinonasal activity according to the BVAS/WG. Four different treatments were analyzed separately so each patient contributed to each analysis and were categorized based on no initiation of treatment (-) vs initiation of treatment (+).. Four medications were evaluated: saline nasal rinse (blue), topical nasal glucocorticoids (orange), topical nasal antibiotic (green), and rituximab within the prior 6 months (RTX; black). No statistically significant differences were found among the four treatment comparisons.
R. Yang: None; E. Romich: None; S. Banerjee: None; N. Amudala: None; P. Merkel: AbbVie/Abbott, 5, Amgen, 2, 5, ArGenx, 2, AstraZeneca, 2, 5, Boehringer-Ingelheim, 2, 5, Bristol-Myers Squibb(BMS), 2, 5, Cabaletta, 2, CSL Behring, 2, Eicos, 5, Electra, 5, Genentech, 5, GlaxoSmithKlein(GSK), 2, 5, HiBio, 2, InflaRx, 2, 5, Janssen, 2, Jubilant, 2, Kyverna, 2, 11, MiroBio, 2, Neutrolis, 5, Novartis, 2, NS Pharma, 2, Q32, 2, Regeneron, 2, Sanofi, 2, Sparrow, 2, Takeda, 2, 5, UpToDate, 9, Visterra, 2; J. Baker: CorEvitas, 2, Cumberland Pharma, 2, Horizon Pharmaceuticals, 5; R. Rhee: None.



