Poster Session B
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (1412–1441) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II: SpA
1421: Domains Impacting Minimal Disease Activity Non-Achievement in Patients with Psoriatic Arthritis and Inadequate Response to TNF Inhibitors Receiving Guselkumab (COSMOS)
Monday, November 13, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall

Laura Coates, MD, PhD
University of Oxford
Oxford, United KingdomDisclosure information not submitted.
Abstract Poster Presenter(s)
Laura Coates1, Carlo Selmi2, Georg Schett3, Pascal Richette4, Felipe Julio Ramirez Garcia5, Wim Noël6, Emmanouil Rampakakis7, Miriam Zimmermann8, Mohamed Sharaf9 and Dennis McGonagle10, 1University of Oxford, Oxford, United Kingdom, 2Rheumatology and Clinical Immunology, Humanitas Research Hospital / Internal Medicine, Humanitas University, Rozzano, Italy, 3Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, 4Lariboisière Hospital, Paris, France, 5Hospital Clínic, Barcelona, Spain, 6Janssen, Zemst, Belgium, 7JSS Medical Research, Saint-Laurent, QC, Canada, 8Janssen Pharmaceuticals, Zug, Switzerland, 9Immunology, Janssen MEA, Dubai, United Arab Emirates, 10Leeds Teaching Hospitals NHS Trust, Academic Unit for the Musculoskeletal Diseases, Leeds, United Kingdom
Background/Purpose: Sustained minimal disease activity (MDA) is achieved by a minority of patients (pts) receiving biologics for psoriatic arthritis (PsA). Pt-reported MDA domains are less frequently achieved than physician-reported domains. Here, we assessed MDA achievement in pts with PsA and inadequate response to 1–2 TNF inhibitors (TNFi-IR) to identify PsA disease domains and factors contributing to the lack of MDA achievement at Week (W)48 for TNFi-IR PsA pts treated with guselkumab (GUS) using data from the Phase 3b COSMOS trial.
Methods: In COSMOS, adults with active PsA (swollen/tender joint counts [SJC/TJC] each ≥3) and TNFi‑IR were randomized 2:1 to subcutaneous GUS 100 mg or placebo (PBO) at W0, W4, then every 8 weeks. PBO pts crossed over to GUS at W16 (early escape) or W24 (planned). MDA was defined as fulfilment of ≥5/7 domains: tender entheses (Leeds enthesitis index [LEI]; 0–6) ≤1; Health Assessment Questionnaire-Disability Index (HAQ-DI; 0–3) ≤0.5; pt pain (0–100) ≤15; Psoriasis Area and Severity Index (PASI; 0–72) ≤1; Pt Global Assessment (PtGA; 0–100) ≤20; SJC (0–66) ≤1; and TJC (0–68) ≤1. Primary fibromyalgia (pFM) was defined at baseline (BL) using TJC minus SJC ≥7 as a proxy. A longitudinal trajectory of achieving each MDA domain through W48 was derived (non-responder imputation). Time to achieving each domain was assessed with Kaplan–Meier analyses; to account for differences in scales and domain strictness, scores were also normalized to the SJC (0–66) scale. Response predictors (for pts not meeting each MDA domain criteria at BL) were identified using multivariate regression for time to achievement (Cox proportional hazards) and W48 achievement (logistic) of MDA.
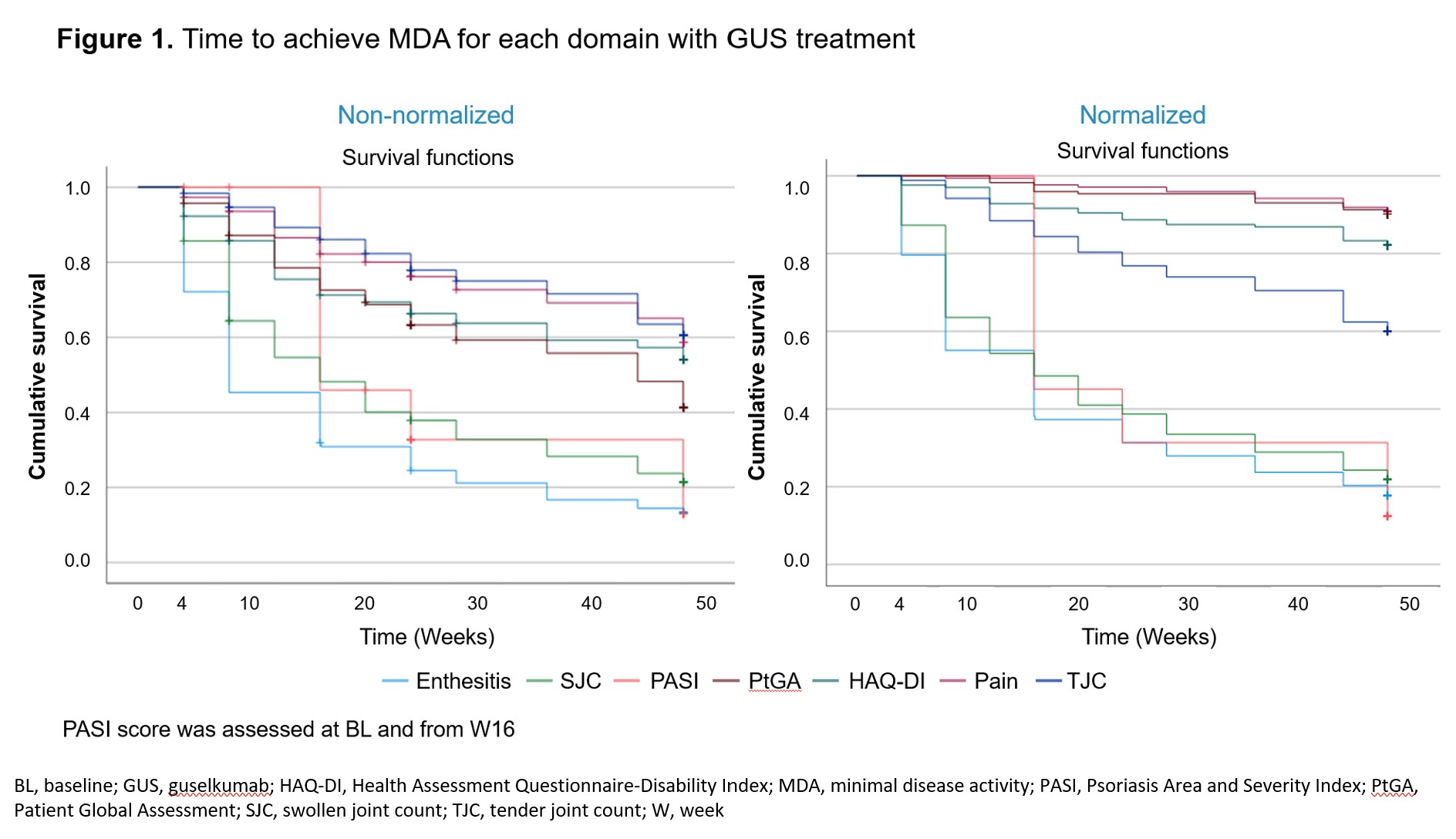
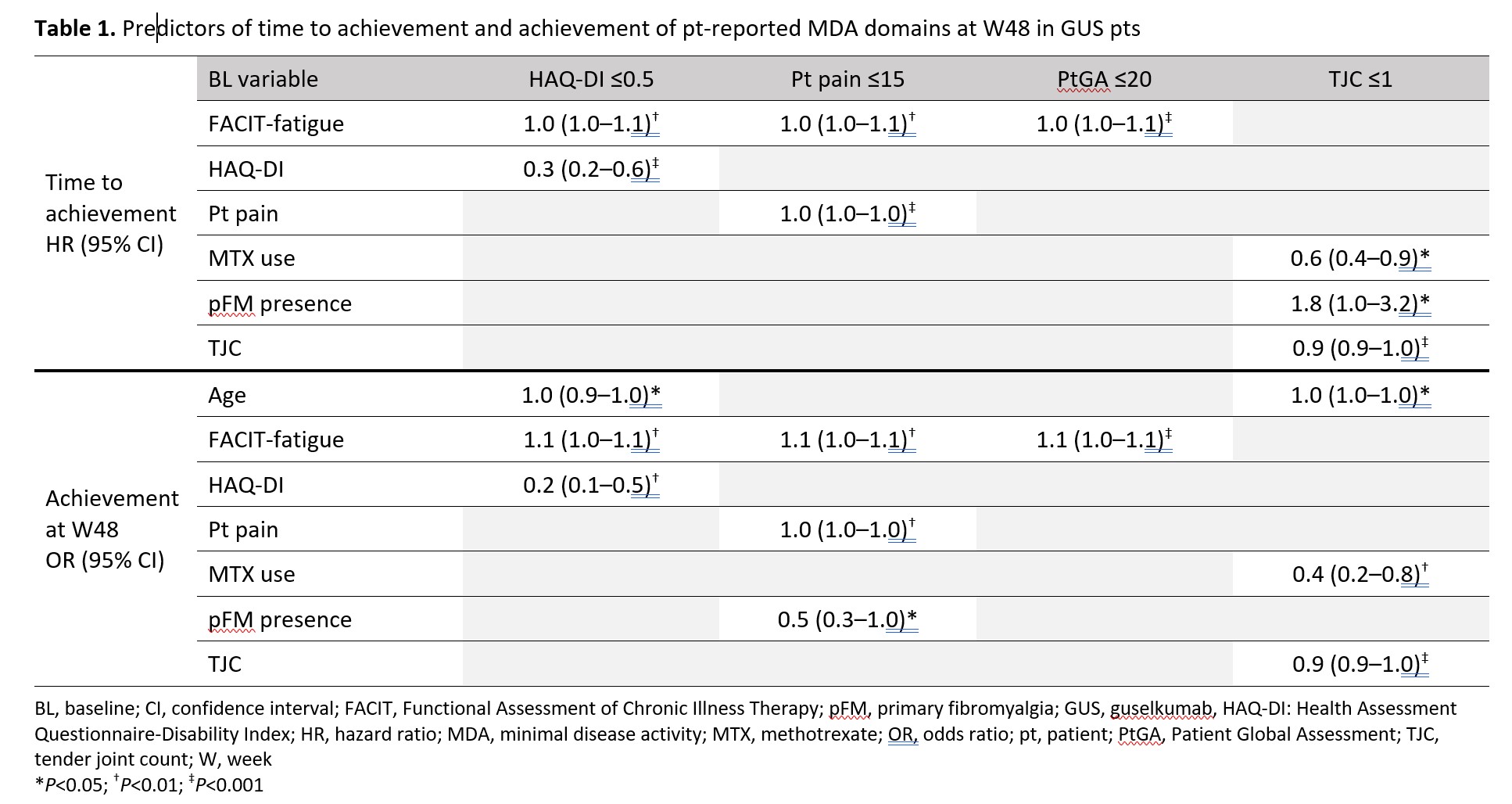
Results: GUS pts (n=189) showed improvement from BL in all MDA domains, with overall W24/48 response rates (%) of: LEI (74.5/79.8), HAQ-DI (26.1/37.0), pt pain (14.7/30.6), PASI (66.8/81.5), PtGA (24.5/39.9), SJC (46.2/63.0), and TJC (14.7/28.3), respectively. Times to achievement of minimal scores for LEI, SJC, and PASI were faster than for PtGA, HAQ-DI, pt pain, and TJC for native-scale scores; when normalized, PtGA, HAQ-DI, and pt pain showed a slower response (Figure 1). Higher BL HAQ-DI and worse fatigue (lower functional assessment of chronic illness therapy [FACIT]-fatigue score) were significantly associated with longer time to HAQ-DI ≤0.5; these factors plus older age predicted W48 non-achievement of HAQ-DI ≤0.5 (Table 1). Worse BL pt pain and fatigue were significant predictors of longer time to pt pain ≤15; these factors plus pFM predicted W48 non-achievement of pt pain ≤15. Worse BL fatigue was also significantly associated with longer time to PtGA ≤20 and W48 non-achievement of PtGA ≤20. Higher TJC, methotrexate (MTX) use, and no pFM at BL were significantly associated with longer time to TJC ≤1; higher BL TJC, MTX use, and older age predicted W48 non-achievement of TJC ≤1.
Conclusion: GUS provided sustainable improvement in all MDA domains through W48. Physician-reported domains (LEI, PASI and SJC) were achieved faster than pt-driven domains (PtGA, HAQ-DI, pt pain, and TJC). BL domain scores, worse fatigue and MTX use (for TJC only) were inversely correlated with MDA in the refractory domains.
L. Coates: AbbVie, 2, 5, 6, Amgen, 2, 5, 6, Biogen, 6, Bristol Myers Squibb, 2, Celgene, 2, 5, 6, Eli Lilly, 2, 5, 6, Galapagos, 2, 6, Gilead Sciences, 2, 6, GSK, 6, Janssen, 2, 5, 6, Medac, 6, MoonLake, 2, Novartis, 2, 5, 6, Pfizer Inc, 2, 5, 6, UCB, 2, 5, 6; C. Selmi: AbbVie, 2, 5, 6, Alfa-Wassermann, 2, 6, Amgen, 2, 5, 6, Biogen, 2, 6, Eli Lilly, 2, 6, Galapagos, 2, 6, Janssen, 2, 6, Novartis, 2, 6, Pfizer, 5, SOBI, 2, 6; G. Schett: None; P. Richette: None; F. Ramirez Garcia: AbbVie/Abbott, 2, 6, Amgen, 6, Eli Lilly, 6, Janssen, 6, 12, Paid Instructor, Novartis, 2, 6, 12, Paid Instructor, Pfizer, 5, 6, UCB, 2, 6; W. Noël: Janssen, 3; E. Rampakakis: Janssen, 2, JSS Medical Research, 3; M. Zimmermann: Janssen, 3; M. Sharaf: Janssen, 3, Johnson & Johnson, 11; D. McGonagle: AbbVie, 2, 5, 6, Celgene, 2, 5, 6, Eli Lilly, 5, 6, 12, Paid Instructor, Janssen, 2, 5, 6, Merck, 2, 5, 6, Novartis, 2, 5, 6, Pfizer, 2, 5, 6, UCB, 2, 5, 6.
Background/Purpose: Sustained minimal disease activity (MDA) is achieved by a minority of patients (pts) receiving biologics for psoriatic arthritis (PsA). Pt-reported MDA domains are less frequently achieved than physician-reported domains. Here, we assessed MDA achievement in pts with PsA and inadequate response to 1–2 TNF inhibitors (TNFi-IR) to identify PsA disease domains and factors contributing to the lack of MDA achievement at Week (W)48 for TNFi-IR PsA pts treated with guselkumab (GUS) using data from the Phase 3b COSMOS trial.
Methods: In COSMOS, adults with active PsA (swollen/tender joint counts [SJC/TJC] each ≥3) and TNFi‑IR were randomized 2:1 to subcutaneous GUS 100 mg or placebo (PBO) at W0, W4, then every 8 weeks. PBO pts crossed over to GUS at W16 (early escape) or W24 (planned). MDA was defined as fulfilment of ≥5/7 domains: tender entheses (Leeds enthesitis index [LEI]; 0–6) ≤1; Health Assessment Questionnaire-Disability Index (HAQ-DI; 0–3) ≤0.5; pt pain (0–100) ≤15; Psoriasis Area and Severity Index (PASI; 0–72) ≤1; Pt Global Assessment (PtGA; 0–100) ≤20; SJC (0–66) ≤1; and TJC (0–68) ≤1. Primary fibromyalgia (pFM) was defined at baseline (BL) using TJC minus SJC ≥7 as a proxy. A longitudinal trajectory of achieving each MDA domain through W48 was derived (non-responder imputation). Time to achieving each domain was assessed with Kaplan–Meier analyses; to account for differences in scales and domain strictness, scores were also normalized to the SJC (0–66) scale. Response predictors (for pts not meeting each MDA domain criteria at BL) were identified using multivariate regression for time to achievement (Cox proportional hazards) and W48 achievement (logistic) of MDA.
Results: GUS pts (n=189) showed improvement from BL in all MDA domains, with overall W24/48 response rates (%) of: LEI (74.5/79.8), HAQ-DI (26.1/37.0), pt pain (14.7/30.6), PASI (66.8/81.5), PtGA (24.5/39.9), SJC (46.2/63.0), and TJC (14.7/28.3), respectively. Times to achievement of minimal scores for LEI, SJC, and PASI were faster than for PtGA, HAQ-DI, pt pain, and TJC for native-scale scores; when normalized, PtGA, HAQ-DI, and pt pain showed a slower response (Figure 1). Higher BL HAQ-DI and worse fatigue (lower functional assessment of chronic illness therapy [FACIT]-fatigue score) were significantly associated with longer time to HAQ-DI ≤0.5; these factors plus older age predicted W48 non-achievement of HAQ-DI ≤0.5 (Table 1). Worse BL pt pain and fatigue were significant predictors of longer time to pt pain ≤15; these factors plus pFM predicted W48 non-achievement of pt pain ≤15. Worse BL fatigue was also significantly associated with longer time to PtGA ≤20 and W48 non-achievement of PtGA ≤20. Higher TJC, methotrexate (MTX) use, and no pFM at BL were significantly associated with longer time to TJC ≤1; higher BL TJC, MTX use, and older age predicted W48 non-achievement of TJC ≤1.
Conclusion: GUS provided sustainable improvement in all MDA domains through W48. Physician-reported domains (LEI, PASI and SJC) were achieved faster than pt-driven domains (PtGA, HAQ-DI, pt pain, and TJC). BL domain scores, worse fatigue and MTX use (for TJC only) were inversely correlated with MDA in the refractory domains.


L. Coates: AbbVie, 2, 5, 6, Amgen, 2, 5, 6, Biogen, 6, Bristol Myers Squibb, 2, Celgene, 2, 5, 6, Eli Lilly, 2, 5, 6, Galapagos, 2, 6, Gilead Sciences, 2, 6, GSK, 6, Janssen, 2, 5, 6, Medac, 6, MoonLake, 2, Novartis, 2, 5, 6, Pfizer Inc, 2, 5, 6, UCB, 2, 5, 6; C. Selmi: AbbVie, 2, 5, 6, Alfa-Wassermann, 2, 6, Amgen, 2, 5, 6, Biogen, 2, 6, Eli Lilly, 2, 6, Galapagos, 2, 6, Janssen, 2, 6, Novartis, 2, 6, Pfizer, 5, SOBI, 2, 6; G. Schett: None; P. Richette: None; F. Ramirez Garcia: AbbVie/Abbott, 2, 6, Amgen, 6, Eli Lilly, 6, Janssen, 6, 12, Paid Instructor, Novartis, 2, 6, 12, Paid Instructor, Pfizer, 5, 6, UCB, 2, 6; W. Noël: Janssen, 3; E. Rampakakis: Janssen, 2, JSS Medical Research, 3; M. Zimmermann: Janssen, 3; M. Sharaf: Janssen, 3, Johnson & Johnson, 11; D. McGonagle: AbbVie, 2, 5, 6, Celgene, 2, 5, 6, Eli Lilly, 5, 6, 12, Paid Instructor, Janssen, 2, 5, 6, Merck, 2, 5, 6, Novartis, 2, 5, 6, Pfizer, 2, 5, 6, UCB, 2, 5, 6.



