Poster Session B
Rheumatoid arthritis (RA)
Session: (1264–1307) RA – Diagnosis, Manifestations, and Outcomes Poster II
1306: Statin Use Attenuates the Impact of Systemic Inflammation on Ischemic Cardiovascular Risk in Patients with Rheumatoid Arthritis
Monday, November 13, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall

George Karpouzas, MD (he/him/his)
Harbor-UCLA Medical Center
Torrance, CA, United StatesDisclosure information not submitted.
Abstract Poster Presenter(s)
George Karpouzas1, Sarah Ormseth2, Piet Van Riel3, Elena Myasoedova4, Miguel A Gonzalez-Gay5, Alfonso Corrales6, Solbritt Rantapaa-Dahlqvist7, Petros Sfikakis8, Patrick Dessein9, Linda Tsang9, Carol Hitchon10, Hani El Gabalawi11, Virginia Pascual Ramos12, Irazú Contreras Yañez13, Iris Colunga14, Dionicio A. Galarza-Delgado14, José Ramón Azpiri-López14, Silvia Rolefstad15, Anne Grete Semb16, Durga P Misra17, George Kitas18 and Ellen-Margrethe Hauge19, 1Harbor-UCLA Medical Center, Torrance, CA, 2The Lundquist Institute, Torrance, CA, 3Radboud University Medical Center, Drunen, Netherlands, 4Mayo Clinic, Rochester, MN, 5IDIVAL and School of Medicine, UC, Santander; Department of Rheumatology, IIS-Fundación Jiménez Díaz, Madrid, Santander, Spain, 6Rheumatology Department, Immunopathology Group, Hospital Universitario Marqués de Valdecilla-IDIVAL, Santander, Spain, 7Umeå University, Umeå, Umea, Sweden, 8National Kapodistrian University of Athens Medical School, Athens, Greece, 9University of Witwatersrand, Johannesburg, South Africa, 10University of Manitoba, Manitoba, MB, Canada, 11University of Manitoba, Winnipeg, MB, Canada, 12Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Mexico City, Mexico, 13Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico, 14Hospital Universitario UANL, Monterrey, Mexico, 15Diakonhjemmet Hospital, Oslo, Norway,, Oslo, Norway, 16Diakonhjemmet Hospital, Oslo, Norway, 17Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGIMS), Lucknow, India, 18The Dudley Group NHS Foundation Trust, Birmingham, United Kingdom, 19Aarhus University Hospital, Aarhus, Denmark
Background/Purpose: Baseline and cumulative inflammation have both been associated with increased cardiovascular event (CVE) risk in patients with rheumatoid arthritis (RA). Statin therapy reduced systemic inflammation, attenuated coronary atherosclerosis progression and promoted plaque calcification and stabilization1 both in general as well as RA patients. We here explored whether baseline statin use influenced the impact of baseline C-reactive protein (CRP) on long-term cardiovascular risk in patients with RA.
Methods: We evaluated 4,357 patients without known cardiovascular disease upon registration to An International Cardiovascular Consortium for people with RA (ATACC-RA) and who were followed prospectively. The primary outcome was ischemic CVE defined as the composite endpoint of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, coronary revascularization, stable angina pectoris, transient ischemic attack, and peripheral arterial disease with or without revascularization. Missing data were imputed using multiple imputation with 10 repetitions. Multivariable Cox models stratified by center evaluated the effect of natural logarithm of CRP, statin use and their interaction on CVE risk after adjusting for age, gender, hypertension, diabetes, family history, smoking, age at RA diagnosis, and total cholesterol to high-density lipoprotein (TC/HDL)ratio. A sensitivity analysis was performed using inverse probability of treatment weights to balance differences between statin treated and untreated patients.
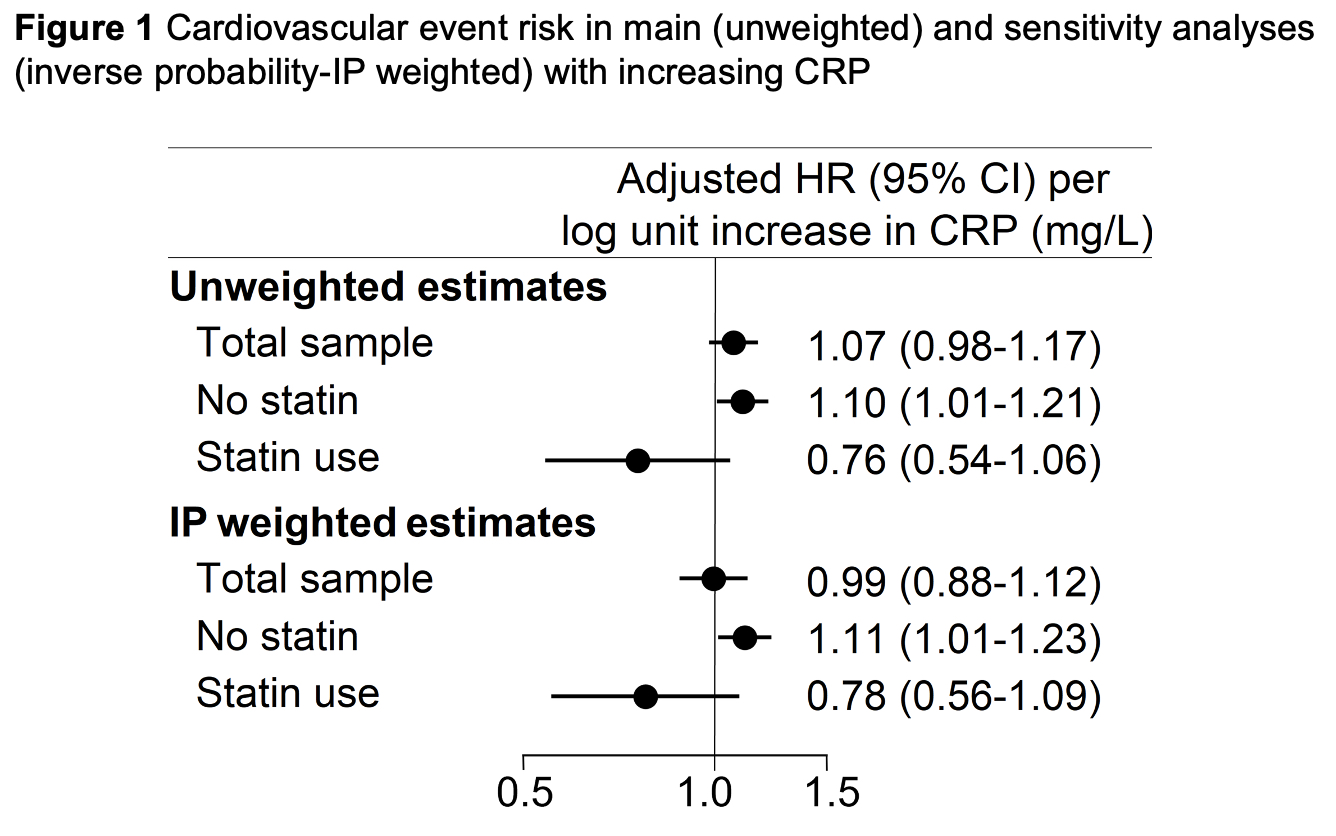
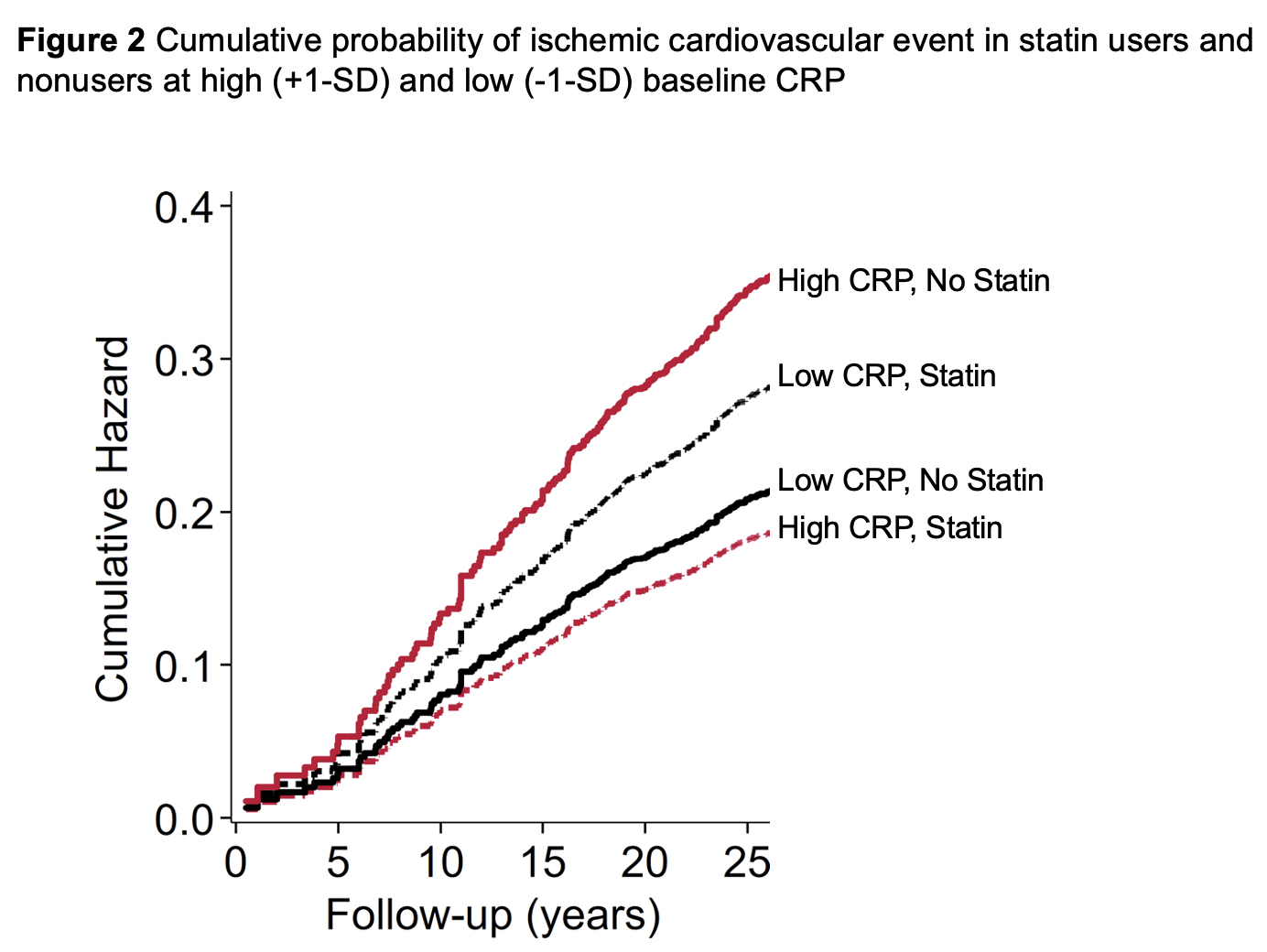
Results: At baseline 462 patients were treated with statins whereas 3,895 were not. Statin therapy inversely associated with low density lipoprotein cholesterol (p< 0.001), TC/HDL ratio (p< 0.001) and CRP(ln) (p=0.048). Over 26,356 patient years (PY) of follow-up, 361 total ischemic CVE were recorded, 321 over 24,235 PY in statin nonusers and 40 over 2,121 PY in statin users. Incidence of any ischemic CVE was 13.3 (95% CI 11.9-14.8)/1000PY among statin nonusers and 18.9 (95% CI 13.8-25.7)/1000PY in statin users (incidence rate difference 5.62 [95% CI -0.41 to 11.64]). In the entire cohort, baseline CRP(ln) was not associated with ischemic CVE risk, [adjusted hazards ratio- aHR 1.07 (95% CI 0.98-1.17), p=0.138]. However, higher CRP(ln) associated with greater risk of the composite outcome exclusively in statin nonusers [aHR 1.10 (95% CI 1.01-1.21), p=0.036] but not in statin users (p-interaction=0.032, Figures 1 and 2). While CRP(ln) was not different between statin groups after inverse probability weighting adjustment (p=0.333), the sensitivity analysis yielded similar results: higher CRP(ln) associated with greater ischemic CVE risk in statin nonusers [aHR 1.11 (95% CI 1.01-1.22), p=0.030] but not among statin users (p-interaction=0.046).
Conclusion: Higher inflammation at baseline associated with greater risk of any ischemic CVE among statin nonusers but not in users. This points to the potential of statin-specific effects directly on atherosclerotic plaque—such as lower progression and stabilization1—above and beyond effects on cholesterol metabolism and systemic inflammation.
Reference: Karpouzas GA et al. Rheumatology (Oxford) 2022;61(5):1857-1866
G. Karpouzas: Janssen, 1, Pfizer, 5, Scipher, 1; S. Ormseth: None; P. Van Riel: None; E. Myasoedova: None; M. Gonzalez-Gay: AbbVie/Abbott, 5, 6, Amgen, 5, 6, Pfizer, 5, 6; A. Corrales: None; S. Rantapaa-Dahlqvist: None; P. Sfikakis: AbbVie/Abbott, 2, 5, Amgen, 2, 5, Boehringer-Ingelheim, 2, 5, Celgene, 2, 5, Eli Lilly, 2, 5, Janssen, 2, 5, Novartis, 2, 5, Pfizer, 2, 5; P. Dessein: None; L. Tsang: None; C. Hitchon: None; H. El Gabalawi: None; V. Pascual Ramos: None; I. Contreras Yañez: None; I. Colunga: None; D. Galarza-Delgado: None; J. Azpiri-López: None; S. Rolefstad: None; A. Semb: None; D. Misra: None; G. Kitas: None; E. Hauge: None.
Background/Purpose: Baseline and cumulative inflammation have both been associated with increased cardiovascular event (CVE) risk in patients with rheumatoid arthritis (RA). Statin therapy reduced systemic inflammation, attenuated coronary atherosclerosis progression and promoted plaque calcification and stabilization1 both in general as well as RA patients. We here explored whether baseline statin use influenced the impact of baseline C-reactive protein (CRP) on long-term cardiovascular risk in patients with RA.
Methods: We evaluated 4,357 patients without known cardiovascular disease upon registration to An International Cardiovascular Consortium for people with RA (ATACC-RA) and who were followed prospectively. The primary outcome was ischemic CVE defined as the composite endpoint of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, coronary revascularization, stable angina pectoris, transient ischemic attack, and peripheral arterial disease with or without revascularization. Missing data were imputed using multiple imputation with 10 repetitions. Multivariable Cox models stratified by center evaluated the effect of natural logarithm of CRP, statin use and their interaction on CVE risk after adjusting for age, gender, hypertension, diabetes, family history, smoking, age at RA diagnosis, and total cholesterol to high-density lipoprotein (TC/HDL)ratio. A sensitivity analysis was performed using inverse probability of treatment weights to balance differences between statin treated and untreated patients.
Results: At baseline 462 patients were treated with statins whereas 3,895 were not. Statin therapy inversely associated with low density lipoprotein cholesterol (p< 0.001), TC/HDL ratio (p< 0.001) and CRP(ln) (p=0.048). Over 26,356 patient years (PY) of follow-up, 361 total ischemic CVE were recorded, 321 over 24,235 PY in statin nonusers and 40 over 2,121 PY in statin users. Incidence of any ischemic CVE was 13.3 (95% CI 11.9-14.8)/1000PY among statin nonusers and 18.9 (95% CI 13.8-25.7)/1000PY in statin users (incidence rate difference 5.62 [95% CI -0.41 to 11.64]). In the entire cohort, baseline CRP(ln) was not associated with ischemic CVE risk, [adjusted hazards ratio- aHR 1.07 (95% CI 0.98-1.17), p=0.138]. However, higher CRP(ln) associated with greater risk of the composite outcome exclusively in statin nonusers [aHR 1.10 (95% CI 1.01-1.21), p=0.036] but not in statin users (p-interaction=0.032, Figures 1 and 2). While CRP(ln) was not different between statin groups after inverse probability weighting adjustment (p=0.333), the sensitivity analysis yielded similar results: higher CRP(ln) associated with greater ischemic CVE risk in statin nonusers [aHR 1.11 (95% CI 1.01-1.22), p=0.030] but not among statin users (p-interaction=0.046).
Conclusion: Higher inflammation at baseline associated with greater risk of any ischemic CVE among statin nonusers but not in users. This points to the potential of statin-specific effects directly on atherosclerotic plaque—such as lower progression and stabilization1—above and beyond effects on cholesterol metabolism and systemic inflammation.
Reference: Karpouzas GA et al. Rheumatology (Oxford) 2022;61(5):1857-1866


G. Karpouzas: Janssen, 1, Pfizer, 5, Scipher, 1; S. Ormseth: None; P. Van Riel: None; E. Myasoedova: None; M. Gonzalez-Gay: AbbVie/Abbott, 5, 6, Amgen, 5, 6, Pfizer, 5, 6; A. Corrales: None; S. Rantapaa-Dahlqvist: None; P. Sfikakis: AbbVie/Abbott, 2, 5, Amgen, 2, 5, Boehringer-Ingelheim, 2, 5, Celgene, 2, 5, Eli Lilly, 2, 5, Janssen, 2, 5, Novartis, 2, 5, Pfizer, 2, 5; P. Dessein: None; L. Tsang: None; C. Hitchon: None; H. El Gabalawi: None; V. Pascual Ramos: None; I. Contreras Yañez: None; I. Colunga: None; D. Galarza-Delgado: None; J. Azpiri-López: None; S. Rolefstad: None; A. Semb: None; D. Misra: None; G. Kitas: None; E. Hauge: None.



