Poster Session B
Rheumatoid arthritis (RA)
Session: (1264–1307) RA – Diagnosis, Manifestations, and Outcomes Poster II
1302: Biomarkers of Cardiovascular Risk in Patients with Rheumatoid Arthritis: Results from the TARGET Trial Biomarker Study
Monday, November 13, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
.png)
Daniel H. Solomon, MD, MPH
Brigham and Women's Hospital
Newton, MA, United StatesDisclosure(s): Janssen: Grant/Research Support (Ongoing)
Abstract Poster Presenter(s)
Daniel Solomon1, Olga Demler2, Pamela Rist2, Leah Santacroce1, Jon Giles3, Katherine Liao1 and Joan Bathon3, 1Brigham and Women's Hospital, Boston, MA, 2Harvard Medical School, Boston, MA, 3Columbia University, New York, NY
Background/Purpose: Cardiovascular (CV) disease remains an important source of morbidity and the most common cause of mortality in patients with rheumatoid arthritis (RA). It has been well recognized that traditional risk models for CV events do not perform well in RA patients. Prior attempts to improve risk prediction have not explored a wide range of candidate biomarkers shown in prior literature to be associated with both RA and CV disease. These analyses tested a wide range of candidate biomarkers against CV risk assessed by arterial inflammation using a FDG PET/CT scan at baseline and 24 weeks.
Methods: These analyses leveraged data from TARGET trial (NCT02374021). Subjects had RA and were inadequate responders to methotrexate (MTX); they were then randomized to add a TNF inhibitor or triple therapy (MTX + sulfasalazine + hydroxychloroquine). Both treatment strategies demonstrated significant suppression of arterial inflammation, measured as the change in target to background ratio in the most diseased segment (MDS TBR) on the FDG PET/CT. However, we observed no significant differences in change in arterial inflammation between the arms. In this analysis, both arms were combined into a single cohort and candidate biomarkers (see Table 1) were tested at baseline and 24 weeks. First, progressively adjusted models tested baseline candidate biomarker values (per standard deviation) as predictors of change in MDS TBR between baseline and 24 weeks. Second, change (also 0 to 24 weeks) in candidate biomarker values (per standard deviation) were tested against change in MDS TBR. Models with only biomarkers, only traditional CV risk factors (Pooled Cohort Equation), and both were tested.
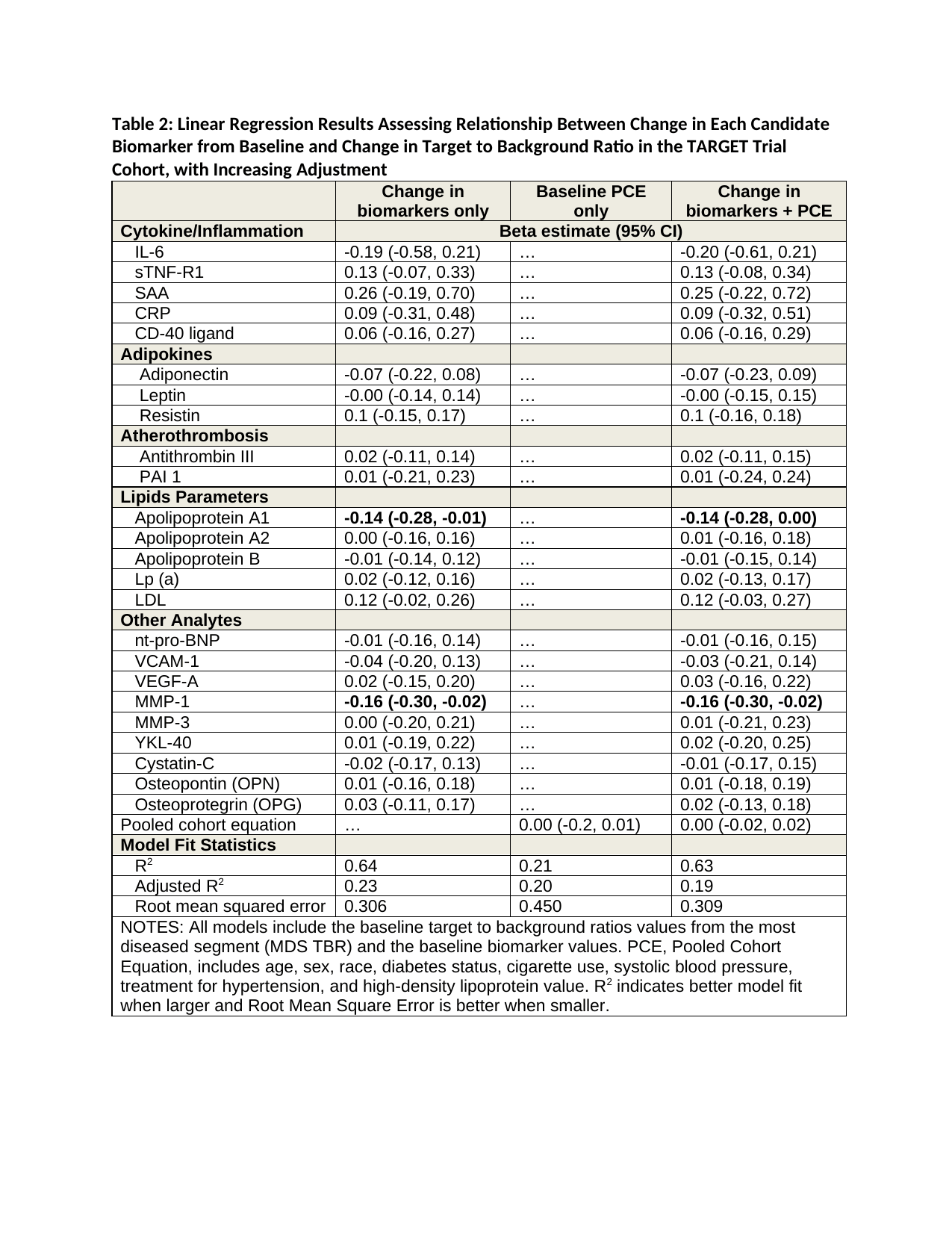
Results: 115 patients had both biomarker data and FDG PET/CT data at baseline and 24 weeks. The patients in TARGET were similar to typical RA patients: 58-year median age; 71% women; 16 months median duration RA; and baseline DAS28 4.8. From the candidate biomarkers tested, we found significant adjusted associations between baseline values of SAA (b = -0.18, 95% CI -0.35, -0.01), CRP (b = 0.19, 95% CI 0.00, 0.38), and YKL-40 (b = 0.14, 95% CI 0.04, 0.25) (see Table 1) and change in arterial inflammation. We found significant adjusted associations between change from baseline to 24 weeks and change in MDS TBR for the following biomarkers (see Table 2): serum apolipoprotein A1 (b = -0.14, 95% CI -0.28, -0.01) and MMP-1 (b = 0.16, 95% CI -0.30, -0.02). Model fit (R2 and Root Mean Square Error, RMSE) was substantially improved when biomarkers were added to traditional CV risk factors.
Conclusion: A broad candidate biomarker scan among patients with RA demonstrated statistically and potentially clinically significant associations with change in arterial inflammation on FDG PET/CT. These biomarkers will be further tested in a large clinical RA cohort with confirmed CV events.

D. Solomon: CorEvitas, 5, Janssen, 5, moderna, 5, Novartis, 5, UpToDate, 9; O. Demler: None; P. Rist: None; L. Santacroce: None; J. Giles: AbbVie, 2, Eli Lilly, 2, Gilead, 2, Novartis, 2, Pfizer, 2; K. Liao: UCB, 2; J. Bathon: None.
Background/Purpose: Cardiovascular (CV) disease remains an important source of morbidity and the most common cause of mortality in patients with rheumatoid arthritis (RA). It has been well recognized that traditional risk models for CV events do not perform well in RA patients. Prior attempts to improve risk prediction have not explored a wide range of candidate biomarkers shown in prior literature to be associated with both RA and CV disease. These analyses tested a wide range of candidate biomarkers against CV risk assessed by arterial inflammation using a FDG PET/CT scan at baseline and 24 weeks.
Methods: These analyses leveraged data from TARGET trial (NCT02374021). Subjects had RA and were inadequate responders to methotrexate (MTX); they were then randomized to add a TNF inhibitor or triple therapy (MTX + sulfasalazine + hydroxychloroquine). Both treatment strategies demonstrated significant suppression of arterial inflammation, measured as the change in target to background ratio in the most diseased segment (MDS TBR) on the FDG PET/CT. However, we observed no significant differences in change in arterial inflammation between the arms. In this analysis, both arms were combined into a single cohort and candidate biomarkers (see Table 1) were tested at baseline and 24 weeks. First, progressively adjusted models tested baseline candidate biomarker values (per standard deviation) as predictors of change in MDS TBR between baseline and 24 weeks. Second, change (also 0 to 24 weeks) in candidate biomarker values (per standard deviation) were tested against change in MDS TBR. Models with only biomarkers, only traditional CV risk factors (Pooled Cohort Equation), and both were tested.
Results: 115 patients had both biomarker data and FDG PET/CT data at baseline and 24 weeks. The patients in TARGET were similar to typical RA patients: 58-year median age; 71% women; 16 months median duration RA; and baseline DAS28 4.8. From the candidate biomarkers tested, we found significant adjusted associations between baseline values of SAA (b = -0.18, 95% CI -0.35, -0.01), CRP (b = 0.19, 95% CI 0.00, 0.38), and YKL-40 (b = 0.14, 95% CI 0.04, 0.25) (see Table 1) and change in arterial inflammation. We found significant adjusted associations between change from baseline to 24 weeks and change in MDS TBR for the following biomarkers (see Table 2): serum apolipoprotein A1 (b = -0.14, 95% CI -0.28, -0.01) and MMP-1 (b = 0.16, 95% CI -0.30, -0.02). Model fit (R2 and Root Mean Square Error, RMSE) was substantially improved when biomarkers were added to traditional CV risk factors.
Conclusion: A broad candidate biomarker scan among patients with RA demonstrated statistically and potentially clinically significant associations with change in arterial inflammation on FDG PET/CT. These biomarkers will be further tested in a large clinical RA cohort with confirmed CV events.


D. Solomon: CorEvitas, 5, Janssen, 5, moderna, 5, Novartis, 5, UpToDate, 9; O. Demler: None; P. Rist: None; L. Santacroce: None; J. Giles: AbbVie, 2, Eli Lilly, 2, Gilead, 2, Novartis, 2, Pfizer, 2; K. Liao: UCB, 2; J. Bathon: None.



