Poster Session B
Periodic fever syndromes, autoinflammatory diseases, Still’s disease and MAS/HLH
Session: (1124–1154) Miscellaneous Rheumatic & Inflammatory Diseases Poster II
1151: Revised IWOS Criteria for Ocular Sarcoidosis: A 2019 Review of the 2009 Criteria in a Study of 384 Patients from a Single University Hospital
Monday, November 13, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- CL
Carmen Lasa Teja, MD
Hospital Universitario Marqués de Valdecilla
Anero, SpainDisclosure information not submitted.
Abstract Poster Presenter(s)
Carmen Lasa1, Jorge Javier Gaitán2, Lara Sanchez-Bilbao3, David Martínez-Lopez4, Inigo Gonzalez-Mazon3, Jose Luis Martin-Varillas5, Rosalía Demetrio2, Raul Fernandez-Ramon2 and Ricardo Blanco6, 1Hospital Universitario Marqués de Valdecilla, IDIVAL., La Cavada, Spain, 2Hospital Universitario Marqués de Valdecilla, Santander, Spain, 3Hospital Universitario Marques de Valdecilla, Santander, Spain, 4Hospital Sierrallana, Torrelavega, Spain, 5Hospital de Laredo, Laredo, Spain, 6Hospital Universitario Marqués de Valdecilla, IDIVAL, Santander, Spain
Background/Purpose: Sarcoidosis is a systemic inflammatory disorder which involves many organs, including eyes. Uveitis and optic neuropathy are the main manifestations. International Workshop on Ocular Sarcoidosis (IWOS) criteria for diagnosis of ocular sarcoidosis (OS) were first published in 2009 and revised in 2019. (question-based survey and panel discussion). IWOS criteria is a remarkable tool to relate uveitis to sarcoidosis, especially when ocular is the first manifestation in the systemic disease. Due to the consequences of OS, identify and initiate appropriate therapy is essential.
Methods: We studied a large cohort (n=384) of all consecutive patients diagnosed with sarcoidosis from January 1, 1999, to December 31, 2019. Finally, 344 patients were included according to the ATS/ERS/WASOG criteria. First (2009) and revised (2019) IWOS criteria were applied to patients diagnosed with Sarcoidosis and ocular symptoms and the results were compared in both groups for our population. Concordance between 2009 and 2019 IWOS criteria was evaluated by calculating Cohen´s kappa coefficient.
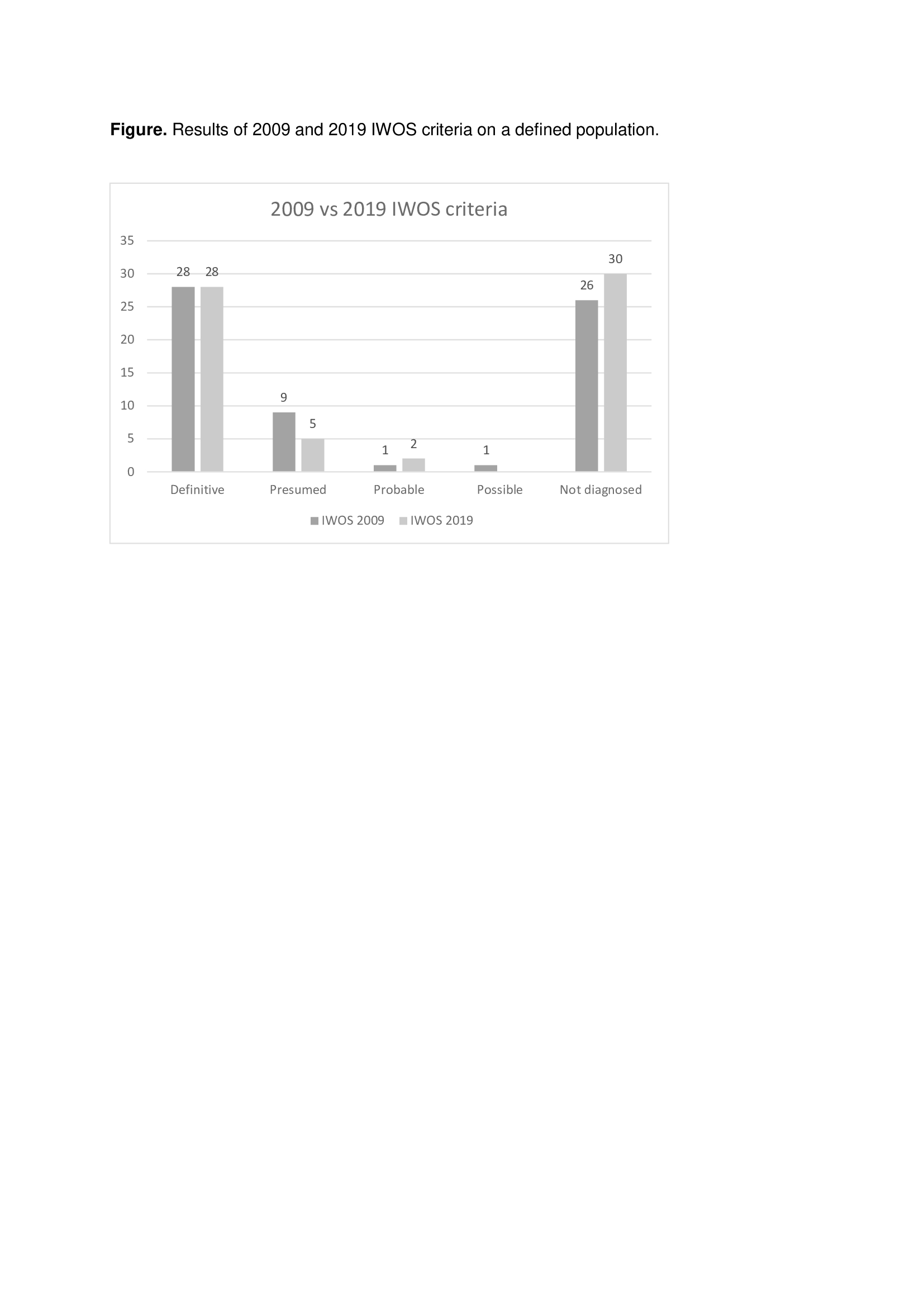
Results: 65 (51% men) of 344 patients had ocular involvement (18.9%), mean age 56.7±16.3 years. As for nationality, 92.3% were Spaniard and 7.7% were South American. A positive biopsy for sarcoidosis was obtained in 75.4% (n=49) and a negative biopsy in 6.2% (n=4). There was no statistically significative difference between diagnostic groups for IWOS 2009/IWOS 2019 in age (p=0.738/p=0.495), sex (p=0.534/p=0.459) nor nationality (p=0.529/p=0.393). When applied 2009 IWOS criteria, 60% (39 patients) met any of the diagnostic categories (43.1% Definitive, 13.8% Presumed, 1.5% Probable, 1.5% Possible). When 2019 IWOS criteria was applied, 53.8% (35 patients) met any of the new diagnostic categories (43.1% Definitive, 7.7% Presumed, 3.1% Probable). Sensitivity for IWOS 2009 was 0.6 and for IWOS 2019 was 0.53. There was statistically significative concordance between 2009 and 2019 IWOS criteria (p< 0.0001) with a strong consistency level (kappa = 0.824). When analyzing IWOS categories separately, we found total concordance in the Definitive category. Probable and Possible categories in 2009 criteria have been merged in only probable in 2019 criteria and there is a total concordance when comparing both. Presumed category represent the bigger change in the revised criteria. We found statistically significative concordance (p=0.000008) with a moderate consistency level (kappa = 0,524).
Conclusion: The revised 2019 IWOS criteria is less sensitive in our population; however, the main difference is in the category Presumed: the requirement of at least 2 intraocular signs of uveitis led to a change of 9 patients (13,8%) in 2009 IWOS down to 5 (7.7%) in 2019. In our population IWOS criteria is still has a low sensitivity.
C. Lasa: None; J. Gaitán: None; L. Sanchez-Bilbao: None; D. Martínez-Lopez: None; I. Gonzalez-Mazon: None; J. Martin-Varillas: None; R. Demetrio: None; R. Fernandez-Ramon: None; R. Blanco: AbbVie, 5, 6, Amgen, 6, AstraZeneca, 2, BMS, 6, Eli Lilly, 6, Galapagos, 2, 6, Janssen, 2, 6, MSD, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche, 5, 6, Sanofi, 6.
Background/Purpose: Sarcoidosis is a systemic inflammatory disorder which involves many organs, including eyes. Uveitis and optic neuropathy are the main manifestations. International Workshop on Ocular Sarcoidosis (IWOS) criteria for diagnosis of ocular sarcoidosis (OS) were first published in 2009 and revised in 2019. (question-based survey and panel discussion). IWOS criteria is a remarkable tool to relate uveitis to sarcoidosis, especially when ocular is the first manifestation in the systemic disease. Due to the consequences of OS, identify and initiate appropriate therapy is essential.
Methods: We studied a large cohort (n=384) of all consecutive patients diagnosed with sarcoidosis from January 1, 1999, to December 31, 2019. Finally, 344 patients were included according to the ATS/ERS/WASOG criteria. First (2009) and revised (2019) IWOS criteria were applied to patients diagnosed with Sarcoidosis and ocular symptoms and the results were compared in both groups for our population. Concordance between 2009 and 2019 IWOS criteria was evaluated by calculating Cohen´s kappa coefficient.
Results: 65 (51% men) of 344 patients had ocular involvement (18.9%), mean age 56.7±16.3 years. As for nationality, 92.3% were Spaniard and 7.7% were South American. A positive biopsy for sarcoidosis was obtained in 75.4% (n=49) and a negative biopsy in 6.2% (n=4). There was no statistically significative difference between diagnostic groups for IWOS 2009/IWOS 2019 in age (p=0.738/p=0.495), sex (p=0.534/p=0.459) nor nationality (p=0.529/p=0.393). When applied 2009 IWOS criteria, 60% (39 patients) met any of the diagnostic categories (43.1% Definitive, 13.8% Presumed, 1.5% Probable, 1.5% Possible). When 2019 IWOS criteria was applied, 53.8% (35 patients) met any of the new diagnostic categories (43.1% Definitive, 7.7% Presumed, 3.1% Probable). Sensitivity for IWOS 2009 was 0.6 and for IWOS 2019 was 0.53. There was statistically significative concordance between 2009 and 2019 IWOS criteria (p< 0.0001) with a strong consistency level (kappa = 0.824). When analyzing IWOS categories separately, we found total concordance in the Definitive category. Probable and Possible categories in 2009 criteria have been merged in only probable in 2019 criteria and there is a total concordance when comparing both. Presumed category represent the bigger change in the revised criteria. We found statistically significative concordance (p=0.000008) with a moderate consistency level (kappa = 0,524).
Conclusion: The revised 2019 IWOS criteria is less sensitive in our population; however, the main difference is in the category Presumed: the requirement of at least 2 intraocular signs of uveitis led to a change of 9 patients (13,8%) in 2009 IWOS down to 5 (7.7%) in 2019. In our population IWOS criteria is still has a low sensitivity.

C. Lasa: None; J. Gaitán: None; L. Sanchez-Bilbao: None; D. Martínez-Lopez: None; I. Gonzalez-Mazon: None; J. Martin-Varillas: None; R. Demetrio: None; R. Fernandez-Ramon: None; R. Blanco: AbbVie, 5, 6, Amgen, 6, AstraZeneca, 2, BMS, 6, Eli Lilly, 6, Galapagos, 2, 6, Janssen, 2, 6, MSD, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche, 5, 6, Sanofi, 6.



