Poster Session B
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (1412–1441) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II: SpA
1434: Long-Term Efficacy and Safety of Risankizumab for Active Psoriatic Arthritis: 148-Week Results from the KEEPsAKE 2 Trial
Monday, November 13, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- FV
Filip Van den Bosch, MD, PhD
Head-of-Clinic and Associate Professor of Rheumatology
Ghent University Hospital
Ghent, BelgiumDisclosure information not submitted.
Abstract Poster Presenter(s)
Andrew Östör1, Filip Van den Bosch2, Kim A Papp3, Cecilia Asnal4, Ricardo Blanco5, Jacob Aelion6, Vassilis Stakias7, Thomas Iyile7, Kyle Carter7, Ahmed Soliman7, Leonidas Drogaris7, Michael Chen7, Byron Padilla7 and Alan Kivitz8, 1Monash University & Emeritus Research; Australian National University, Melbourne, Australia, 2Department of Internal Medicine and Pediatrics, Ghent University and VIB Center for Inflammation Research, Ghent, Belgium, 3Alliance Clinical Research and Probity Medical Research, Waterloo, and University of Toronto, Toronto, ON, Canada, 4Hospital Alemán, Buenos Aires, Argentina, 5Rheumatology Department, Immunopathology Group, Hospital Universitario Marqués de Valdecilla-IDIVAL, Santander, Spain, 6West Tennessee Research Institute, Jackson, TN, 7AbbVie, Inc., North Chicago, IL, 8Altoona Center for Clinical Research, Duncansville, PA
Background/Purpose: PsA is a chronic systemic inflammatory disease that affects 30% of patients diagnosed with psoriasis, with a clinical burden that includes dactylitis, enthesitis, cutaneous manifestations, chronic pain, progressive joint damage, and disability. Risankizumab (RZB), an antibody that targets the p19 subunit of interleukin-23 with high affinity and specificity, is approved for the treatment of adult patients with active PsA. RZB has been previously reported to show efficacy across several disease domains up to week 100.1 Here, we report the efficacy and safety results through week 148.
Methods: KEEPsAKE 2 is an ongoing phase 3 global, multicenter clinical trial evaluating the efficacy and safety of RZB versus placebo (PBO) in patients with active PsA,defined as ≥5 tender joints and ≥5 swollen joints, meeting the Classification Criteria for Psoriatic Arthritis (CASPAR), with symptom onset of ≥6 months before screening, and active plaque psoriasis or nail changes consistent with psoriasis at screening. Eligible patients were 18 years or older and had previous inadequate response or intolerance to 1 or 2 biological therapies (Bio-IR) and/or ≥1 conventional synthetic DMARD (csDMARD-IR). Patients were randomized in a 1:1 ratio to receive double-blinded treatment with subcutaneous RZB 150 mg or matched placebo for 24 weeks, administered at weeks 0, 4 and 16. Starting at week 24, all patients in the ongoing trial receive open-label RZB 150 mg every 12 weeks through week 316. Efficacy and safety analyses were conducted in all randomized patients who received one or more doses of the study drug. Statistical reporting and imputation methods for efficacy assessments are defined in the figures. Safety assessments were based on monitoring of treatment emergent adverse events (TEAEs) and are summarized using exposure-adjusted event rates (EAERs, events/100 patient-years [PYs]).
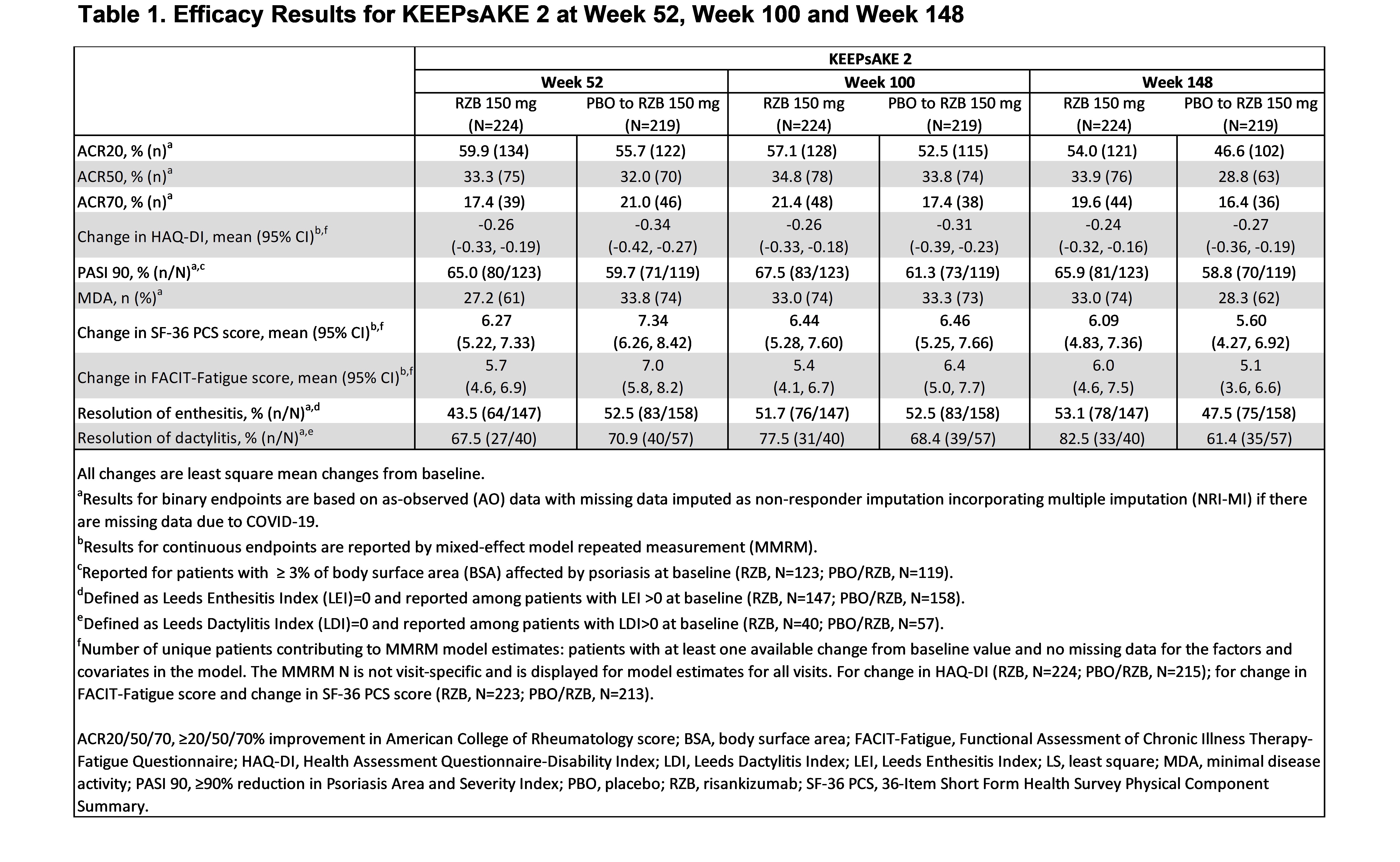
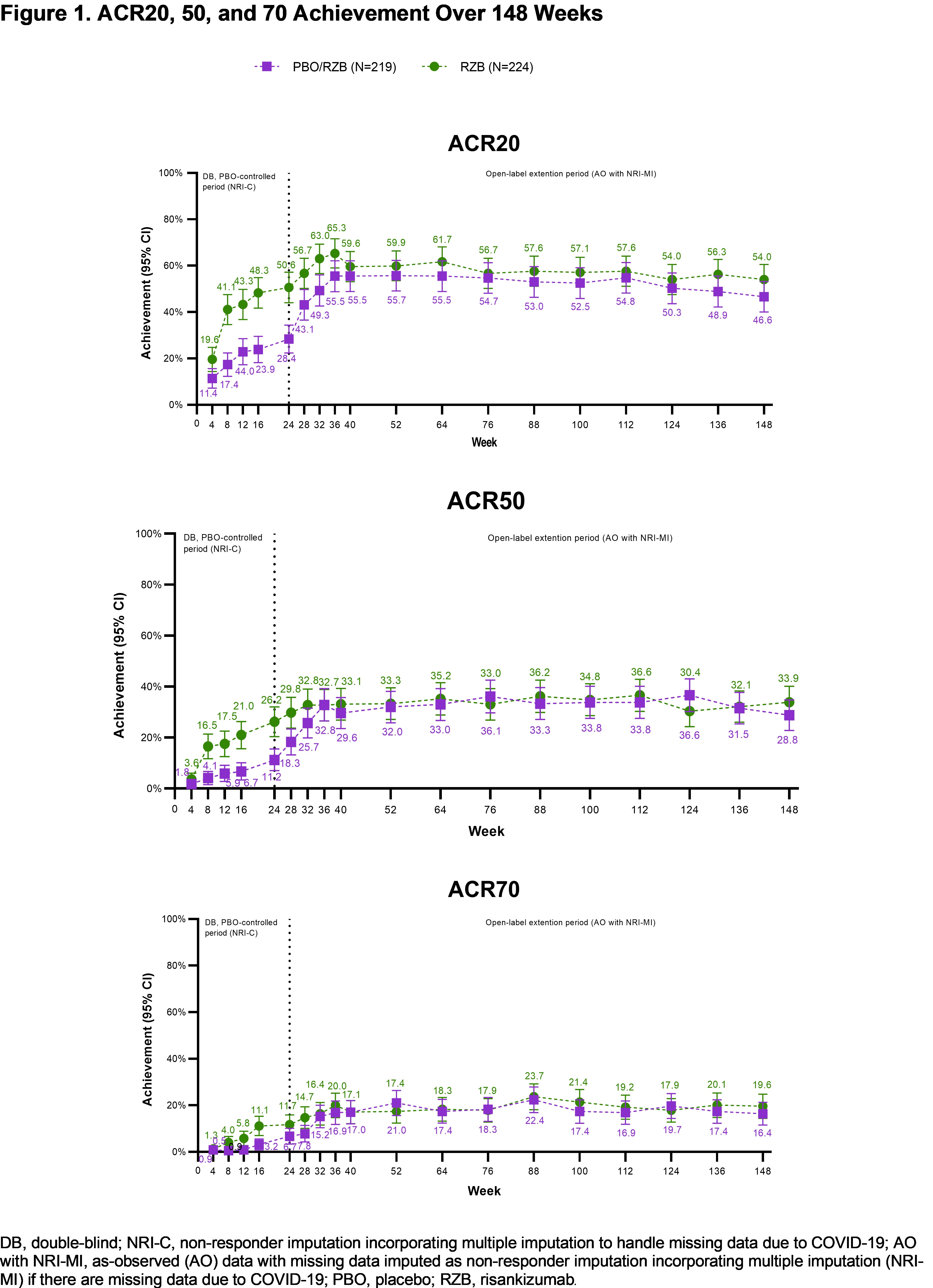
Results: Patients in KEEPsAKE 2 (RZB N=224; PBO/RZB N=219) maintained similar efficacy results at week 148 to those reported at week 52 and week 100 (Table 1). 33.9% of RZB and 28.8% of PBO/RZB patients achieved ACR50 response at week 148 (Figure 1). 65.9% of RZB and 58.8% of PBO/RZB patients achieved PASI 90 response at week 148. A consistent change from baseline in HAQ-DI (RZB -0.24, PBO/RZB -0.27), SF-36 PCS (RZB 6.09, PBO/RZB 5.60) and FACIT-Fatigue (RZB 6.0, PBO/RZB 5.1) was observed at 148 weeks. 33.0% of RZB patients and 28.3% of PBO/RZB patients achieved MDA at week 148, consistent with results reported at week 52 and week 100. For those patients with enthesitis at baseline, resolution was observed in 53.1% of RZB and 47.5% of PBO/RZB patients at week 148. For patients with dactylitis at baseline, resolution was observed in 82.5% of RZB and 61.4% of RZB/PBO patients at week 148. The overall rates of TEAEs, serious TEAEs and AEs leading to discontinuation of study drug remained stable and was consistent with the rates reported for the placebo-controlled period (Table 2).
Conclusion: Long-term treatment with RZB 150mg shows durable efficacy in patients with PsA through 148 weeks, with no new safety findings.
References:
.jpg)
A. Östör: AbbVie, 12, has served as a consultant and/or on advisory boards and/or undertaken clinical trials, GSK, 12, has served as a consultant and/or on advisory boards and/or undertaken clinical trials, Janssen, 12, has served as a consultant and/or on advisory boards and/or undertaken clinical trials, Lilly, 12, has served as a consultant and/or on advisory boards and/or undertaken clinical trials, Novartis, 12, has served as a consultant and/or on advisory boards and/or undertaken clinical trials, Pfizer, 12, has served as a consultant and/or on advisory boards and/or undertaken clinical trials; F. Van den Bosch: AbbVie, 2, 6, Amgen, 2, BMS, 6, Celgene, 6, Eli Lilly, 2, Galapagos, 2, Janssen, 2, 6, Merck, 2, 6, Novartis, 2, 6, Pfizer, 2, 6, UCB Pharma, 2, 6; K. Papp: AbbVie, 1, 2, 5, 6, Akros, 1, 2, 5, 6, Amgen, 1, 2, 5, 6, Anacor, 1, 2, 5, 6, Arcutis, 1, 2, 5, 6, Astellas, 1, 2, 5, 6, Bausch Health/Valeant, 1, 2, 5, 6, Baxalta, 1, 2, 5, 6, Boehringer-Ingelheim, 1, 2, 5, 6, Bristol-Myers Squibb, 1, 2, 5, 6, Can-Fite Biopharma, 1, 2, 5, 6, Celgene, 1, 2, 5, 6, Coherus, 1, 2, 5, 6, Dermira, 1, 2, 5, 6, Dow Pharma, 1, 2, 5, 6, Eli Lilly, 1, 2, 5, 6, Evelo, 1, 2, 5, 6, Forward Pharma, 5, Galapagos, 1, 2, 5, 6, Galderma, 1, 2, 5, 6, Genentech, 1, 2, 5, 6, Gilead, 1, 2, 5, 6, GlaxoSmithKlein, 1, 2, 5, 6, Janssen, 1, 2, 5, 6, Kyowa-Hakko Kirin, 1, 2, 5, 6, LEO Pharma, 1, 2, 5, 6, MedImmune, 1, 2, 5, 6, Meiji Seika Pharma, 1, 2, 5, 6, Merck-Serono, 1, 2, 5, 6, Mitsubishi Pharma, 1, 2, 5, 6, Moberg Pharma, 1, 2, 5, 6, MSD, 1, 2, 5, 6, Novartis, 1, 2, 5, 6, Pfizer, 1, 2, 5, 6, PRCL Research, 1, 2, 5, 6, Regeneron, 1, 2, 5, 6, Roche, 1, 2, 5, 6, Sanofi-Aventis/Genzyme, 1, 2, 5, 6, Sun Pharma, 1, 2, 5, 6, Takeda, 1, 2, 5, 6, UCB, 1, 2, 5, 6; C. Asnal: AbbVie/Abbott, 1, 5, 6, Amgen, 1, 5, 6, Eli Lilly, 1, 5, 6, Genentech, 1, 5, 6, Janssen, 1, 5, 6, Novartis, 1, 5, 6, Pfizer, 1, 5, 6, Roche, 1, 5, 6, R-Pharm, 1, 5, 6; R. Blanco: AbbVie/Abbott, 5, 6, Amgen, 6, AstraZeneca, 2, Bristol-Myers Squibb(BMS), 6, Eli Lilly, 6, Galapagos, 2, 6, Janssen, 2, 6, Merck/MSD, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche, 5, 6, Sanofi, 6; J. Aelion: AbbVie, 5, 6, Acceleron, 5, Acelyrin, 5, Aclaris Therapeutics, 5, Alpine Immune Sciences, 5, Amgen, 2, 5, AstraZeneca, 5, Biogen, 5, Bristol Myers Squibb, 2, 5, Eli Lilly, 5, Galapagos, 5, GlaxoSmithKline, 5, Horizon, 5, Janssen, 2, 5, Novartis, 2, 5, Roche, 5, Selecta, 5, UCB, 5, Ventyx, 5; V. Stakias: AbbVie, 3, 11; T. Iyile: AbbVie/Abbott, 3, 11; K. Carter: AbbVie/Abbott, 3, 11; A. Soliman: AbbVie/Abbott, 3, 10, 11; L. Drogaris: AbbVie/Abbott, 3, 11; M. Chen: AbbVie/Abbott, 3, 11; B. Padilla: AbbVie/Abbott, 3, 11; A. Kivitz: AbbVie, 6, Amgen, 6, 11, Chemocentryx, 1, Eli Lilly, 6, Fresenius Kabi, 2, Genzyme, 2, Gilead, 2, 11, GlaxoSmithKlein (GSK), 2, 6, 11, Grunenthal, 2, Horizon, 1, 2, Janssen, 1, 2, Novartis, 4, 11, Pfizer, 2, 6, 11, Selecta, 2, Synact, 2, Takeda, 2, UCB, 1, 6.
Background/Purpose: PsA is a chronic systemic inflammatory disease that affects 30% of patients diagnosed with psoriasis, with a clinical burden that includes dactylitis, enthesitis, cutaneous manifestations, chronic pain, progressive joint damage, and disability. Risankizumab (RZB), an antibody that targets the p19 subunit of interleukin-23 with high affinity and specificity, is approved for the treatment of adult patients with active PsA. RZB has been previously reported to show efficacy across several disease domains up to week 100.1 Here, we report the efficacy and safety results through week 148.
Methods: KEEPsAKE 2 is an ongoing phase 3 global, multicenter clinical trial evaluating the efficacy and safety of RZB versus placebo (PBO) in patients with active PsA,defined as ≥5 tender joints and ≥5 swollen joints, meeting the Classification Criteria for Psoriatic Arthritis (CASPAR), with symptom onset of ≥6 months before screening, and active plaque psoriasis or nail changes consistent with psoriasis at screening. Eligible patients were 18 years or older and had previous inadequate response or intolerance to 1 or 2 biological therapies (Bio-IR) and/or ≥1 conventional synthetic DMARD (csDMARD-IR). Patients were randomized in a 1:1 ratio to receive double-blinded treatment with subcutaneous RZB 150 mg or matched placebo for 24 weeks, administered at weeks 0, 4 and 16. Starting at week 24, all patients in the ongoing trial receive open-label RZB 150 mg every 12 weeks through week 316. Efficacy and safety analyses were conducted in all randomized patients who received one or more doses of the study drug. Statistical reporting and imputation methods for efficacy assessments are defined in the figures. Safety assessments were based on monitoring of treatment emergent adverse events (TEAEs) and are summarized using exposure-adjusted event rates (EAERs, events/100 patient-years [PYs]).
Results: Patients in KEEPsAKE 2 (RZB N=224; PBO/RZB N=219) maintained similar efficacy results at week 148 to those reported at week 52 and week 100 (Table 1). 33.9% of RZB and 28.8% of PBO/RZB patients achieved ACR50 response at week 148 (Figure 1). 65.9% of RZB and 58.8% of PBO/RZB patients achieved PASI 90 response at week 148. A consistent change from baseline in HAQ-DI (RZB -0.24, PBO/RZB -0.27), SF-36 PCS (RZB 6.09, PBO/RZB 5.60) and FACIT-Fatigue (RZB 6.0, PBO/RZB 5.1) was observed at 148 weeks. 33.0% of RZB patients and 28.3% of PBO/RZB patients achieved MDA at week 148, consistent with results reported at week 52 and week 100. For those patients with enthesitis at baseline, resolution was observed in 53.1% of RZB and 47.5% of PBO/RZB patients at week 148. For patients with dactylitis at baseline, resolution was observed in 82.5% of RZB and 61.4% of RZB/PBO patients at week 148. The overall rates of TEAEs, serious TEAEs and AEs leading to discontinuation of study drug remained stable and was consistent with the rates reported for the placebo-controlled period (Table 2).
Conclusion: Long-term treatment with RZB 150mg shows durable efficacy in patients with PsA through 148 weeks, with no new safety findings.
References:
- Kristensen, et al. 2022 EADV Congress.

.jpg)

A. Östör: AbbVie, 12, has served as a consultant and/or on advisory boards and/or undertaken clinical trials, GSK, 12, has served as a consultant and/or on advisory boards and/or undertaken clinical trials, Janssen, 12, has served as a consultant and/or on advisory boards and/or undertaken clinical trials, Lilly, 12, has served as a consultant and/or on advisory boards and/or undertaken clinical trials, Novartis, 12, has served as a consultant and/or on advisory boards and/or undertaken clinical trials, Pfizer, 12, has served as a consultant and/or on advisory boards and/or undertaken clinical trials; F. Van den Bosch: AbbVie, 2, 6, Amgen, 2, BMS, 6, Celgene, 6, Eli Lilly, 2, Galapagos, 2, Janssen, 2, 6, Merck, 2, 6, Novartis, 2, 6, Pfizer, 2, 6, UCB Pharma, 2, 6; K. Papp: AbbVie, 1, 2, 5, 6, Akros, 1, 2, 5, 6, Amgen, 1, 2, 5, 6, Anacor, 1, 2, 5, 6, Arcutis, 1, 2, 5, 6, Astellas, 1, 2, 5, 6, Bausch Health/Valeant, 1, 2, 5, 6, Baxalta, 1, 2, 5, 6, Boehringer-Ingelheim, 1, 2, 5, 6, Bristol-Myers Squibb, 1, 2, 5, 6, Can-Fite Biopharma, 1, 2, 5, 6, Celgene, 1, 2, 5, 6, Coherus, 1, 2, 5, 6, Dermira, 1, 2, 5, 6, Dow Pharma, 1, 2, 5, 6, Eli Lilly, 1, 2, 5, 6, Evelo, 1, 2, 5, 6, Forward Pharma, 5, Galapagos, 1, 2, 5, 6, Galderma, 1, 2, 5, 6, Genentech, 1, 2, 5, 6, Gilead, 1, 2, 5, 6, GlaxoSmithKlein, 1, 2, 5, 6, Janssen, 1, 2, 5, 6, Kyowa-Hakko Kirin, 1, 2, 5, 6, LEO Pharma, 1, 2, 5, 6, MedImmune, 1, 2, 5, 6, Meiji Seika Pharma, 1, 2, 5, 6, Merck-Serono, 1, 2, 5, 6, Mitsubishi Pharma, 1, 2, 5, 6, Moberg Pharma, 1, 2, 5, 6, MSD, 1, 2, 5, 6, Novartis, 1, 2, 5, 6, Pfizer, 1, 2, 5, 6, PRCL Research, 1, 2, 5, 6, Regeneron, 1, 2, 5, 6, Roche, 1, 2, 5, 6, Sanofi-Aventis/Genzyme, 1, 2, 5, 6, Sun Pharma, 1, 2, 5, 6, Takeda, 1, 2, 5, 6, UCB, 1, 2, 5, 6; C. Asnal: AbbVie/Abbott, 1, 5, 6, Amgen, 1, 5, 6, Eli Lilly, 1, 5, 6, Genentech, 1, 5, 6, Janssen, 1, 5, 6, Novartis, 1, 5, 6, Pfizer, 1, 5, 6, Roche, 1, 5, 6, R-Pharm, 1, 5, 6; R. Blanco: AbbVie/Abbott, 5, 6, Amgen, 6, AstraZeneca, 2, Bristol-Myers Squibb(BMS), 6, Eli Lilly, 6, Galapagos, 2, 6, Janssen, 2, 6, Merck/MSD, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche, 5, 6, Sanofi, 6; J. Aelion: AbbVie, 5, 6, Acceleron, 5, Acelyrin, 5, Aclaris Therapeutics, 5, Alpine Immune Sciences, 5, Amgen, 2, 5, AstraZeneca, 5, Biogen, 5, Bristol Myers Squibb, 2, 5, Eli Lilly, 5, Galapagos, 5, GlaxoSmithKline, 5, Horizon, 5, Janssen, 2, 5, Novartis, 2, 5, Roche, 5, Selecta, 5, UCB, 5, Ventyx, 5; V. Stakias: AbbVie, 3, 11; T. Iyile: AbbVie/Abbott, 3, 11; K. Carter: AbbVie/Abbott, 3, 11; A. Soliman: AbbVie/Abbott, 3, 10, 11; L. Drogaris: AbbVie/Abbott, 3, 11; M. Chen: AbbVie/Abbott, 3, 11; B. Padilla: AbbVie/Abbott, 3, 11; A. Kivitz: AbbVie, 6, Amgen, 6, 11, Chemocentryx, 1, Eli Lilly, 6, Fresenius Kabi, 2, Genzyme, 2, Gilead, 2, 11, GlaxoSmithKlein (GSK), 2, 6, 11, Grunenthal, 2, Horizon, 1, 2, Janssen, 1, 2, Novartis, 4, 11, Pfizer, 2, 6, 11, Selecta, 2, Synact, 2, Takeda, 2, UCB, 1, 6.



