Poster Session B
Rheumatoid arthritis (RA)
Session: (1308–1344) RA – Treatment Poster II
1327: Effects of Long-Term Low Dose Glucocorticoid Treatment for Rheumatoid Arthritis on Body Weight and Blood Pressure: A Pooled Analysis of Individual Patient Data from Five Randomised Trials
Monday, November 13, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- AP
Andriko Palmowski, MD
Charité - Universitätsmedizin Berlin
Berlin, GermanyDisclosure information not submitted.
Abstract Poster Presenter(s)
Andriko Palmowski1, Sabrina Mai Nielsen2, Zhivana Boyadzhieva3, Linda Hartman4, Judith Oldenkott5, Björn Svensson6, Ingiäld Hafström7, Siegfried Wassenberg8, Ernest Choy9, John Kirwan10, Robin Christensen11, Maarten Boers12 and Frank Buttgereit13, 1Charité - Universitätsmedizin Berlin, Berlin, Germany, 2The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark, 3Charité Universitatsmedizine - Berlin, Berlin, Germany, 4Amsterdam University Medical Centers, Amsterdam, Netherlands, 5Charite - University Medicine Berlin, Berlin, Germany, 6Lund University, Lund, Sweden, 7Karolinska Institutet, Stockholm, Sweden, 8Rheumazentrum Ratingen, Ratingen, Germany, 9Section of Rheumatology, Cardiff University, Cardiff, United Kingdom, 10University of Bristol, Bristol, United Kingdom, 11Musculoskeletal Statistics Unit, The Parker Institute, Copenhagen, Denmark, 12Amsterdam UMC, Vrije Universiteit, Amersfoort, Netherlands, 13Charité Universitätsmedizin, Dept. Rheumatology, Berlin, Germany
Background/Purpose: High-dose glucocorticoids (GCs) can cause weight gain and hypertension. It is unclear whether GCs at ≤7.5mg/day prednisone equivalent ("low dose"), administered for rheumatoid arthritis (RA), do as well. Prior studies could not definitively answer this research question: Observational studies are confounded by indication, and individual randomized trials are usually underpowered for small safety signals. The objective was to assess the effects of long-term treatment with low dose GCs in RA on body weight and blood pressure by pooling individual patient data from randomized trials.
Methods: Data from five randomised controlled trials with two-year interventions were pooled.1-5 Intervention groups received GCs at a dose of ≤7.5mg/day prednisone equivalent. Co-primary outcomes were the difference in change from baseline in a) body weight (kg) and b) mean arterial blood pressure (MAP; mmHg). A secondary outcome was difference in the change of number of administered antihypertensive drugs. Several sensitivity and subgroup analyses were conducted. All analyses were based on analyses of covariance, trial ID was included as a random effects factor to account for clustering of patients within trials. Multiple imputation was used to account for missing data under the Intention-To-Treat approach. No imputations were performed for trials with no data collected or available for the given outcome. A detailed prespecified protocol (dx.doi.org/10.17504/protocols.io.x54v9y4d1g3e/v1) with a gatekeeping procedure for statistical testing was followed. All trials originated in Europe (twelve countries), and all allowed concomitant treatment with disease-modifying antirheumatic drugs.
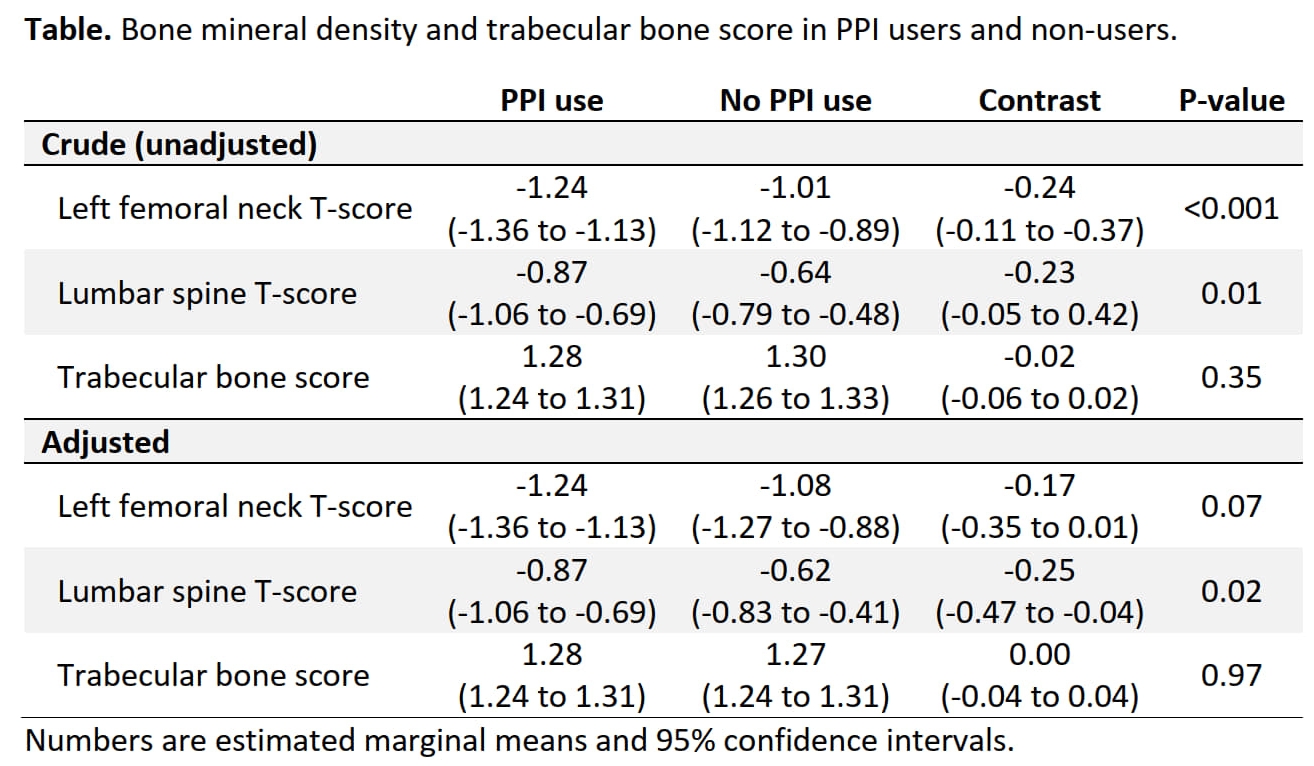
Results: 1,112 participants were included (mean ± SD age 61 ± 15 years; 68% female). A mean DAS28 of 4.87 ± 1.16 indicates moderate disease activity at baseline. Disability was moderate to severe with a median (interquartile range) health assessment questionnaire score of 1.38 (0.80; 2.25). 64% and 67% of patients with available data were ACPA and rheumatoid factor positive, respectively, and the median (interquartile range) disease duration was 1 year (0.42; 7). Most patients (44%) were never smokers. Baseline values for weight and MAP were 73kg ± 14 and 98mmHg ± 12; median number of antihypertensive drugs was 0 (interquartile range 0; 1). After two years, patients on GCs gained mean 1.1kg (95%CI 0.5 to 1.8; p < 0.001; Table) more weight than patients in the control groups. Both groups increased MAP by 2-3mmHg, without difference between the groups (–0.4; 95% CI –3.0 to 2.2 mm Hg; p = 0.19; Table). The change in number of antihypertensive drugs was low and similar in both groups. Results were consistent across sensitivity and subgroup analyses focusing on overweight or hypertensive patients, and when comparing GCs at 5mg/d with 7.5mg/d (data not shown).
Conclusion: We present robust evidence that low dose GCs, taken over two years for the treatment of RA, lead to about one additional kg of weight gain but do not cause changes in blood pressure.
References: 1 Boers et al. Ann Rheum Dis 2022 2 Kirwan et al. NEJM 1995 3 Choy et al. Ann Rheum Dis 2005 4 Wassenberg et al. Arthritis Rheumatol 2005 5 Svensson Arthritis Rheumatol 2005
A. Palmowski: None; S. Nielsen: None; Z. Boyadzhieva: None; L. Hartman: None; J. Oldenkott: None; B. Svensson: None; I. Hafström: None; S. Wassenberg: AbbVie/Abbott, 6, Eli Lilly, 2, 6, Galapagos, 2, Medac, 6, Merck/MSD, 6, Pfizer, 5, 6, UCB, 2; E. Choy: AbbVie, 2, 6, Amgen, 2, 6, Bio-Cancer, 5, Biogen, 2, 5, Chugai Pharma, 2, 6, Eli Lilly, 2, 6, Fresenius Kabi, 2, 6, Galapagos, 2, 6, Gilead, 2, 6, Janssen, 2, Novartis, 5, Pfizer, 5, 6, R-Pharm, 2, Sanofi, 2, 5, 6, Sanofi-Genzyme, 2, UCB, 2; J. Kirwan: None; R. Christensen: None; M. Boers: Celltrion, 2, 6, Novartis, 2, 6, Pfizer, 2, 6; F. Buttgereit: AbbVie/Abbott, 6, Horizon Therapeutics, 5, Pfizer, 5, 6, Roche, 6.
Background/Purpose: High-dose glucocorticoids (GCs) can cause weight gain and hypertension. It is unclear whether GCs at ≤7.5mg/day prednisone equivalent ("low dose"), administered for rheumatoid arthritis (RA), do as well. Prior studies could not definitively answer this research question: Observational studies are confounded by indication, and individual randomized trials are usually underpowered for small safety signals. The objective was to assess the effects of long-term treatment with low dose GCs in RA on body weight and blood pressure by pooling individual patient data from randomized trials.
Methods: Data from five randomised controlled trials with two-year interventions were pooled.1-5 Intervention groups received GCs at a dose of ≤7.5mg/day prednisone equivalent. Co-primary outcomes were the difference in change from baseline in a) body weight (kg) and b) mean arterial blood pressure (MAP; mmHg). A secondary outcome was difference in the change of number of administered antihypertensive drugs. Several sensitivity and subgroup analyses were conducted. All analyses were based on analyses of covariance, trial ID was included as a random effects factor to account for clustering of patients within trials. Multiple imputation was used to account for missing data under the Intention-To-Treat approach. No imputations were performed for trials with no data collected or available for the given outcome. A detailed prespecified protocol (dx.doi.org/10.17504/protocols.io.x54v9y4d1g3e/v1) with a gatekeeping procedure for statistical testing was followed. All trials originated in Europe (twelve countries), and all allowed concomitant treatment with disease-modifying antirheumatic drugs.
Results: 1,112 participants were included (mean ± SD age 61 ± 15 years; 68% female). A mean DAS28 of 4.87 ± 1.16 indicates moderate disease activity at baseline. Disability was moderate to severe with a median (interquartile range) health assessment questionnaire score of 1.38 (0.80; 2.25). 64% and 67% of patients with available data were ACPA and rheumatoid factor positive, respectively, and the median (interquartile range) disease duration was 1 year (0.42; 7). Most patients (44%) were never smokers. Baseline values for weight and MAP were 73kg ± 14 and 98mmHg ± 12; median number of antihypertensive drugs was 0 (interquartile range 0; 1). After two years, patients on GCs gained mean 1.1kg (95%CI 0.5 to 1.8; p < 0.001; Table) more weight than patients in the control groups. Both groups increased MAP by 2-3mmHg, without difference between the groups (–0.4; 95% CI –3.0 to 2.2 mm Hg; p = 0.19; Table). The change in number of antihypertensive drugs was low and similar in both groups. Results were consistent across sensitivity and subgroup analyses focusing on overweight or hypertensive patients, and when comparing GCs at 5mg/d with 7.5mg/d (data not shown).
Conclusion: We present robust evidence that low dose GCs, taken over two years for the treatment of RA, lead to about one additional kg of weight gain but do not cause changes in blood pressure.
References: 1 Boers et al. Ann Rheum Dis 2022 2 Kirwan et al. NEJM 1995 3 Choy et al. Ann Rheum Dis 2005 4 Wassenberg et al. Arthritis Rheumatol 2005 5 Svensson Arthritis Rheumatol 2005

Table. Changes in weight and mean arterial blood pressure in GC and control groups over two years.
A. Palmowski: None; S. Nielsen: None; Z. Boyadzhieva: None; L. Hartman: None; J. Oldenkott: None; B. Svensson: None; I. Hafström: None; S. Wassenberg: AbbVie/Abbott, 6, Eli Lilly, 2, 6, Galapagos, 2, Medac, 6, Merck/MSD, 6, Pfizer, 5, 6, UCB, 2; E. Choy: AbbVie, 2, 6, Amgen, 2, 6, Bio-Cancer, 5, Biogen, 2, 5, Chugai Pharma, 2, 6, Eli Lilly, 2, 6, Fresenius Kabi, 2, 6, Galapagos, 2, 6, Gilead, 2, 6, Janssen, 2, Novartis, 5, Pfizer, 5, 6, R-Pharm, 2, Sanofi, 2, 5, 6, Sanofi-Genzyme, 2, UCB, 2; J. Kirwan: None; R. Christensen: None; M. Boers: Celltrion, 2, 6, Novartis, 2, 6, Pfizer, 2, 6; F. Buttgereit: AbbVie/Abbott, 6, Horizon Therapeutics, 5, Pfizer, 5, 6, Roche, 6.



