Poster Session B
Systemic lupus erythematosus (SLE)
Session: (1442–1487) SLE – Diagnosis, Manifestations, & Outcomes Poster II
1461: Association of Serum Analytes with SLE Cognitive Impairment Phenotypes Formed by Machine Learning: MMP-9, S100A8/A9, IL-6, IL-10, and NGAL
Monday, November 13, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- CM
Carolina Munoz-Grajales, MD, PhD (she/her/hers)
UHN/TWH
Toronto, ON, CanadaDisclosure information not submitted.
Abstract Poster Presenter(s)
Michelle Barraclough1, Carolina Munoz-Grajales2, Lauren Erdman3, Juan Pablo Diaz Martinez4, Kathleen Bingham1, Mahta Kakvan5, Roberta Kretzmann6, Carmela Tartaglia7, Lesley Ruttan8, May Choi9, Simone Appenzeller10, Sherief Marzouk6, Dennisse Bonilla4, Patti Katz11, Dorcas Beaton12, Anna Goldenberg13, Robin Green6, Joan Wither1 and Zahi Touma6, 1University Health Network, Toronto, ON, Canada, 2UHN/TWH, Toronto, ON, Canada, 3Hospital for SickKids, Toronto, Toronto, ON, Canada, 4Schroeder Arthritis Institute, University Health Network, Toronto, ON, Canada, 5University Health Network, University of Toronto, Toronto, ON, Canada, 6University of Toronto, Toronto, ON, Canada, 7Krembil Brain Institute, University Health Network, University of Toronto, Toronto, ON, Canada, 8University Health Network, Toronto Rehabilitation Institute, Toronto, ON, Canada, 9University of Calgary, Calgary, AB, Canada, 10UNICAMP, Campinas, Brazil, 11University of California San Francisco, San Rafael, CA, 12Institute for Work & Health, Toronto, ON, Canada, 13Hospital for SickKids, University of Toronto, Toronto, ON, Canada
Background/Purpose: Cognitive impairment (CI) is highly prevalent in patients with SLE [prevalence of 38% (range: 20%-80%)]. The exact mechanisms underlying CI is complex and multifactorial. Understanding the relationship between SLE CI phenotypes and analytes may be crucial for improving patient care and developing targeted interventions. We have previously defined two SLE CI subtypes (A and B) where subtype A performed worst on objective cognitive function compared with subtype B. Subtype A also, had greater levels of disease burden/damage, worse performance on subjective cognitive function, worse HRQoL and psychiatric measures compared with subtype B. We aimed to explore the associations between SLE CI phenotypes and serum analytes levels.
Methods: SLE patients aged 18-65 years attending a single lupus centre (January 2016 – October 2019) completed the ACR Neuropsychological Battery (ACR-NB) cognitive assessment. Age and gender matched normative data were used to obtain z-scores on all 19 tests of ACR-NB. The ACR-NB tests were reduced using principal component analysis (PCA). Similarity network fusion (SNF) was used to identify patient subtypes on the ACR-NB data, demographic and clinical variables, disease burden/activity, health related quality of life (HRQoL: SF-36, LupusQoL), the PDQ-20 (perceived cognitive deficits), Beck Depression Inventory-II, Beck Anxiety Inventory, and the fatigue severity scale (FSS) in addition to the serum levels of nine analytes (IL-6, IL-10, IFN-ɣ, MMP-9, NGAL/lipocalin, S100A8/A9, S100B, TNF-α, and TWEAK [determined by ELISA]). Differences between the SNF identified subtypes were evaluated using Kruskal-Wallis tests and chi-square tests.
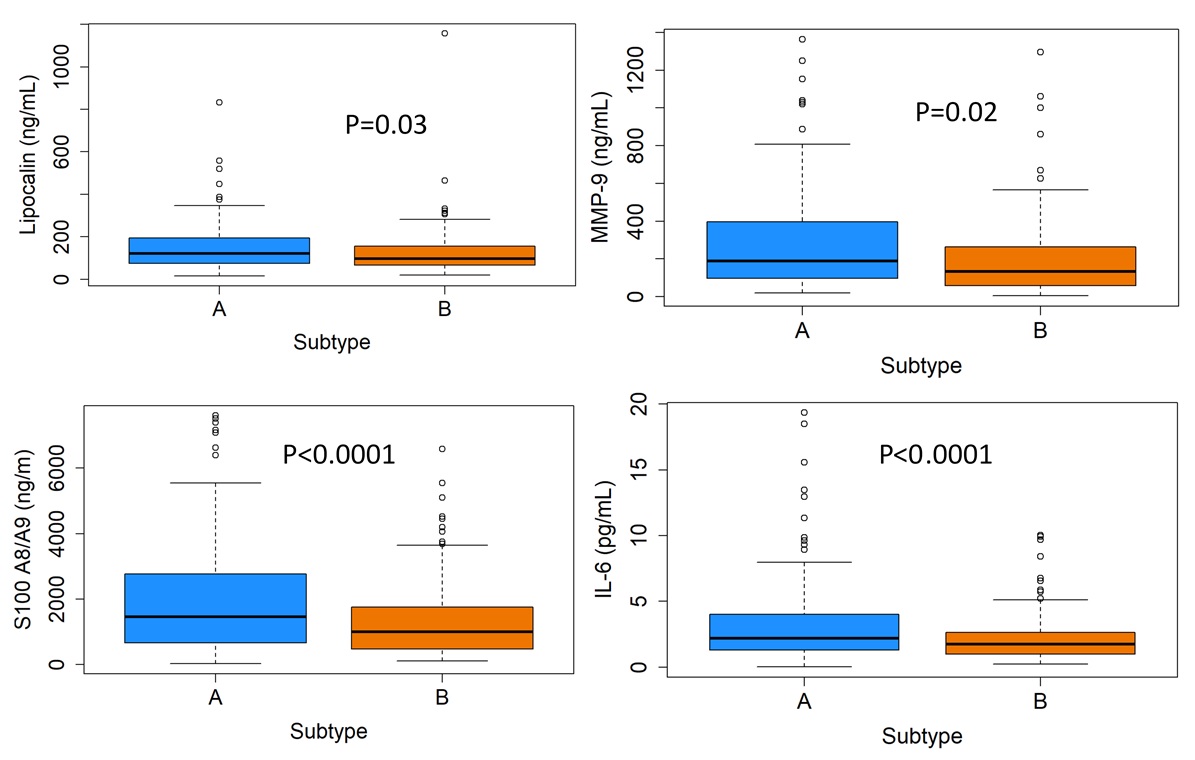
Results: Of the 296 patients, 87% were female, mean age 41.5 ± 18.4 and mean disease duration 13.8 ± 10.1 years at study visit.The level of S100A8/A9, MMP-9, NGAL/lipocalin, and IL-6 were statistically significantly higher in the more severe SLE CI subtype A compared to B (Figure 1). No difference in the levels of IL-10, IFN-ɣ, S100B, TNF-α, and TWEAK were identified between SLE CI subtypes A and B.
Conclusion: This study demonstrated a higher level of serum analytes in association with the SLE CI subtypes identified with machine learning analysis. S100A8/A9, MMP-9, NGAL, and IL-6 levels were higher in the more severe subtype A where patients experience worse objective and and subjective cognitive function with a higher disease burden and damage compared with subtype B. The results of this study will further in deciphering the mechanisms of cognitive impairment in patients with SLE and the identification of targeted therapy.
M. Barraclough: None; C. Munoz-Grajales: None; L. Erdman: None; J. Diaz Martinez: None; K. Bingham: None; M. Kakvan: None; R. Kretzmann: None; C. Tartaglia: None; L. Ruttan: None; M. Choi: AbbVie/Abbott, 2, 6, Amgen, 2, 6, AstraZeneca, 2, 6, Bristol-Myers Squibb(BMS), 2, 6, Celgene, 2, 6, Eli Lilly, 2, 6, GlaxoSmithKlein(GSK), 2, Janssen, 2, 6, Mallinckrodt, 2, Merck/MSD, 2, MitogenDx, 2, Organon, 6, Pfizer, 2, 6, Roche, 2, Werfen, 2; S. Appenzeller: None; S. Marzouk: None; D. Bonilla: None; P. Katz: None; D. Beaton: None; A. Goldenberg: None; R. Green: None; J. Wither: AstraZeneca, 1, 6, Pfizer, 12, Indirect salary support through a Chair award to the Division of Rheumatology at the University of Toronto; Z. Touma: AstraZeneca, 2, GSK, 2.
Background/Purpose: Cognitive impairment (CI) is highly prevalent in patients with SLE [prevalence of 38% (range: 20%-80%)]. The exact mechanisms underlying CI is complex and multifactorial. Understanding the relationship between SLE CI phenotypes and analytes may be crucial for improving patient care and developing targeted interventions. We have previously defined two SLE CI subtypes (A and B) where subtype A performed worst on objective cognitive function compared with subtype B. Subtype A also, had greater levels of disease burden/damage, worse performance on subjective cognitive function, worse HRQoL and psychiatric measures compared with subtype B. We aimed to explore the associations between SLE CI phenotypes and serum analytes levels.
Methods: SLE patients aged 18-65 years attending a single lupus centre (January 2016 – October 2019) completed the ACR Neuropsychological Battery (ACR-NB) cognitive assessment. Age and gender matched normative data were used to obtain z-scores on all 19 tests of ACR-NB. The ACR-NB tests were reduced using principal component analysis (PCA). Similarity network fusion (SNF) was used to identify patient subtypes on the ACR-NB data, demographic and clinical variables, disease burden/activity, health related quality of life (HRQoL: SF-36, LupusQoL), the PDQ-20 (perceived cognitive deficits), Beck Depression Inventory-II, Beck Anxiety Inventory, and the fatigue severity scale (FSS) in addition to the serum levels of nine analytes (IL-6, IL-10, IFN-ɣ, MMP-9, NGAL/lipocalin, S100A8/A9, S100B, TNF-α, and TWEAK [determined by ELISA]). Differences between the SNF identified subtypes were evaluated using Kruskal-Wallis tests and chi-square tests.
Results: Of the 296 patients, 87% were female, mean age 41.5 ± 18.4 and mean disease duration 13.8 ± 10.1 years at study visit.The level of S100A8/A9, MMP-9, NGAL/lipocalin, and IL-6 were statistically significantly higher in the more severe SLE CI subtype A compared to B (Figure 1). No difference in the levels of IL-10, IFN-ɣ, S100B, TNF-α, and TWEAK were identified between SLE CI subtypes A and B.
Conclusion: This study demonstrated a higher level of serum analytes in association with the SLE CI subtypes identified with machine learning analysis. S100A8/A9, MMP-9, NGAL, and IL-6 levels were higher in the more severe subtype A where patients experience worse objective and and subjective cognitive function with a higher disease burden and damage compared with subtype B. The results of this study will further in deciphering the mechanisms of cognitive impairment in patients with SLE and the identification of targeted therapy.

Figure 1
Subtype A (blue) performed worst on objective cognitive function compared with subtype B. Subtype A also, had greater levels of disease burden/damage, worse performance on subjective cognitive function, worse HRQoL and psychiatric measures compared with subtype B (orange).
Subtype A (blue) performed worst on objective cognitive function compared with subtype B. Subtype A also, had greater levels of disease burden/damage, worse performance on subjective cognitive function, worse HRQoL and psychiatric measures compared with subtype B (orange).
M. Barraclough: None; C. Munoz-Grajales: None; L. Erdman: None; J. Diaz Martinez: None; K. Bingham: None; M. Kakvan: None; R. Kretzmann: None; C. Tartaglia: None; L. Ruttan: None; M. Choi: AbbVie/Abbott, 2, 6, Amgen, 2, 6, AstraZeneca, 2, 6, Bristol-Myers Squibb(BMS), 2, 6, Celgene, 2, 6, Eli Lilly, 2, 6, GlaxoSmithKlein(GSK), 2, Janssen, 2, 6, Mallinckrodt, 2, Merck/MSD, 2, MitogenDx, 2, Organon, 6, Pfizer, 2, 6, Roche, 2, Werfen, 2; S. Appenzeller: None; S. Marzouk: None; D. Bonilla: None; P. Katz: None; D. Beaton: None; A. Goldenberg: None; R. Green: None; J. Wither: AstraZeneca, 1, 6, Pfizer, 12, Indirect salary support through a Chair award to the Division of Rheumatology at the University of Toronto; Z. Touma: AstraZeneca, 2, GSK, 2.



