Poster Session B
Systemic lupus erythematosus (SLE)
Session: (1442–1487) SLE – Diagnosis, Manifestations, & Outcomes Poster II
1455: Risk Factors of Cytomegalovirus Reactivation and Disease in Patients with Systemic Lupus Erythematosus
Monday, November 13, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- MW
Miyu Wakatsuki, MD
National Center for Global Health and Medicine
Sinjuku-ku, Toyama, 1-21-1, JapanDisclosure information not submitted.
Abstract Poster Presenter(s)
Miyu Wakatsuki1, Hiroyuki Yamashita1, Yuuya Akiyama1, Setsuko Oyama1, Shintarou Aozaki1, Ryo Kuwata2, Misa Yamaji1, Takuya Harada1, Kyouko Motomura1, Yuusuke Nakamichi1 and Hiroshi Kaneko3, 1National Center for Global Health and Medicine, Tokyo, Japan, 2Department of Rheumatology, National Center for Global Health and Medicine, Tokyo, Japan, 3Division of Rheumatic Disease, National Center for Global Health and Medicine, Tokyo, Japan
Background/Purpose: Cytomegalovirus (CMV) infection is classified as an opportunistic infection that occurs in autoimmune diseases. Systemic lupus erythematosus (SLE) is one of the most frequently reported autoimmune diseases that cause CMV reactivation, but no studies have examined the risk factors in patients with SLE. This study aimed to identify the risk factors of CMV reactivation and disease in patients with SLE undergoing remission induction therapy.
Methods: This study reviewed patients with SLE who received remission induction therapy at our institution from May 2010 to October 2022 and enrolled patients whose CMV pp65 antigen levels were measured within 3 months of admission. Patients with CMV reactivation were divided into two groups, namely, CMV disease (presence of symptoms or end-organ disease) and asymptomatic CMV reactivation. We examined the risk factors associated with CMV reactivation and disease.
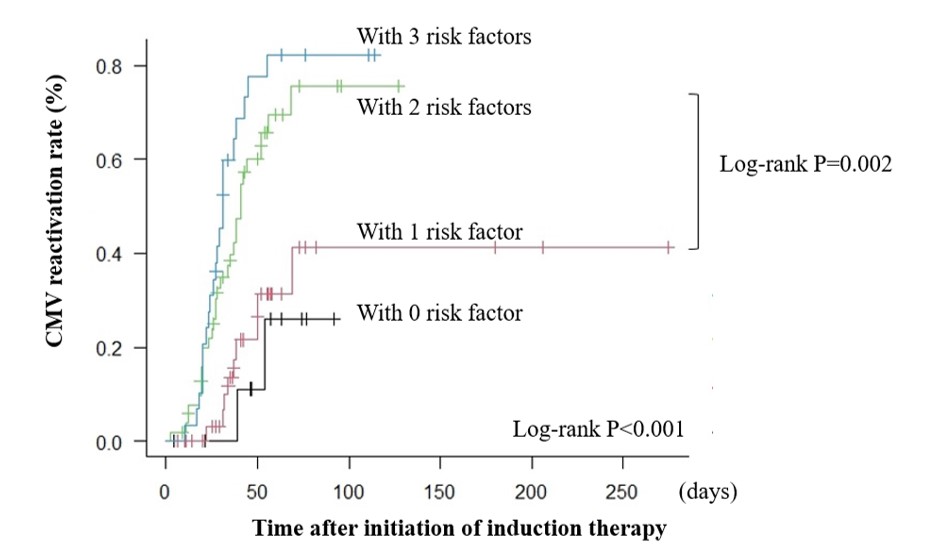
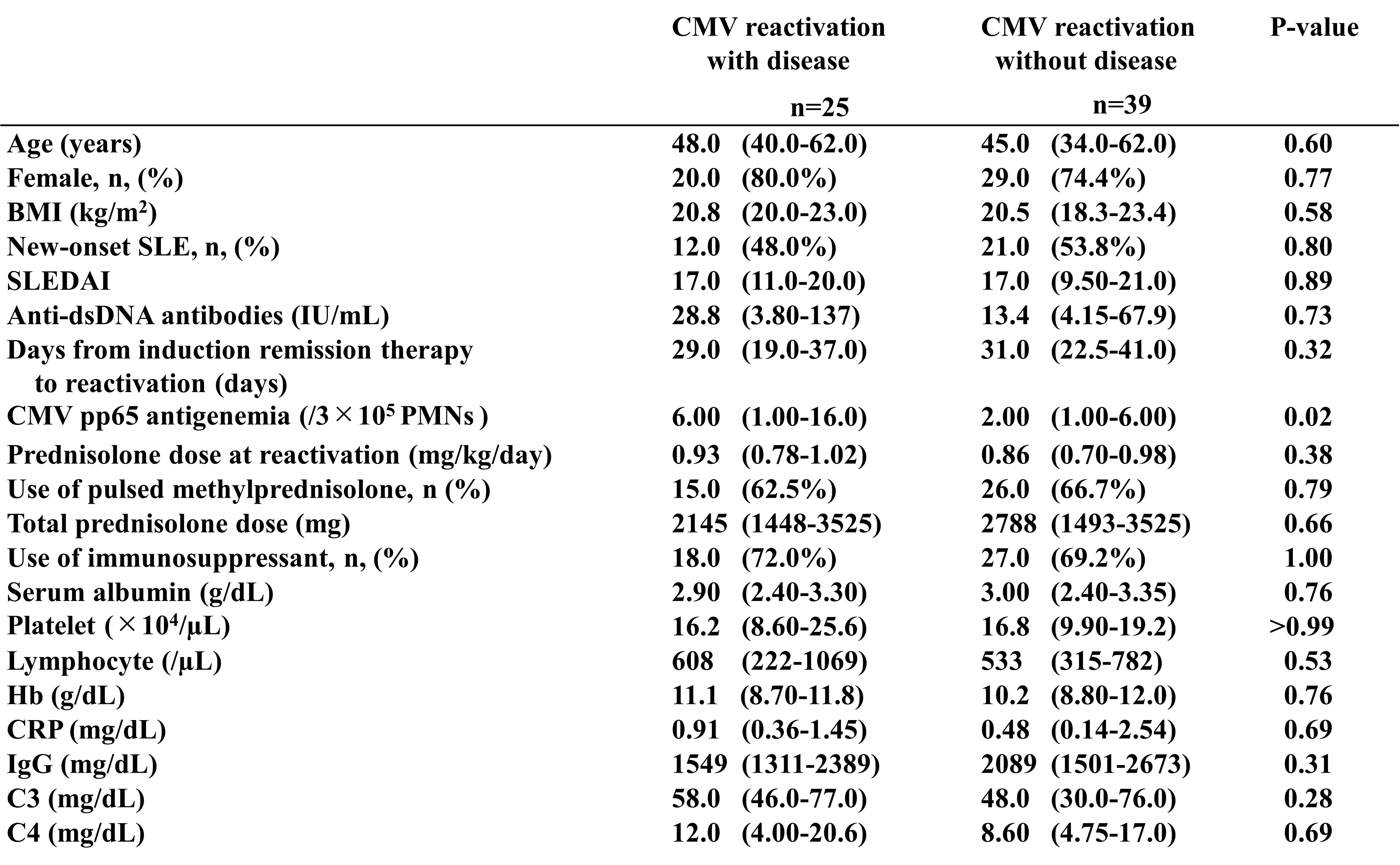
Results: CMV reactivation was observed in 64 out of 130 patients. Univariate analysis revealed the association between CMV reactivation and old age (46.5 vs. 39.5; P = 0.02), lower serum albumin levels at admission (2.9 vs. 3.3; P< 0.001), higher Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) (17 vs. 10; P = 0.001) at admission, and increased steroid pulse therapy (64.1% vs. 45.5%; P = 0.04) and immunosuppressive drug use (70.3% vs. 50.0%; P = 0.02) (Table 1). Log-rank tests on the above factors revealed significant differences for all except for steroid pulse therapy. Moreover, the Cox proportional hazards regression analysis on the four factors revealed the association of old age (hazard ratio [HR]: 2.03, 95% confidence interval [CI]: 1.09–3.78, P = 0.026), lower albumin (HR: 2.35, 95% CI: 1.09–5.07, P = 0.029), higher SLEDAI (HR: 1.73, 95% CI: 1.00–2.98, P = 0.048), and immunosuppressive drug use (HR: 1.94, 95% CI: 1.12–3.35, P = 0.018) with CMV reactivation. Receiver operating characteristic (ROC) curve analysis using the above four factors revealed that the sensitivity and specificity were 82.8% and 60.6%, respectively (area under the curve = 0.748, 95% CI: 0.664–0.831) for CMV reactivation when all four factors were present. The log-rank test revealed that CMV reactivation developed more frequently when two or more risk factors were present (Figure 1). Among the 64 cases with CMV reactivation, an increased number of CMV pp65 antigen-positive cells was found in the 25 cases with CMV disease than the 39 cases without the disease (6 vs. 2; P = 0.02) (Table 2). ROC curve analysis revealed that the cutoff value for organ damage development was 12 cells out of 2 slides (sensitivity: 36.0% and specificity: 94.9%).
Conclusion: Old age, low albumin levels, high disease activity, and the use of immunosuppressive drugs are possible risk factors of CMV reactivation in patients with SLE. CMV pp65 antigen-positive cells of 12 per 2 slides were determined for the diagnosis of CMV disease.
.jpg)
M. Wakatsuki: None; H. Yamashita: None; Y. Akiyama: None; S. Oyama: None; S. Aozaki: None; R. Kuwata: None; M. Yamaji: None; T. Harada: None; K. Motomura: None; Y. Nakamichi: None; H. Kaneko: None.
Background/Purpose: Cytomegalovirus (CMV) infection is classified as an opportunistic infection that occurs in autoimmune diseases. Systemic lupus erythematosus (SLE) is one of the most frequently reported autoimmune diseases that cause CMV reactivation, but no studies have examined the risk factors in patients with SLE. This study aimed to identify the risk factors of CMV reactivation and disease in patients with SLE undergoing remission induction therapy.
Methods: This study reviewed patients with SLE who received remission induction therapy at our institution from May 2010 to October 2022 and enrolled patients whose CMV pp65 antigen levels were measured within 3 months of admission. Patients with CMV reactivation were divided into two groups, namely, CMV disease (presence of symptoms or end-organ disease) and asymptomatic CMV reactivation. We examined the risk factors associated with CMV reactivation and disease.
Results: CMV reactivation was observed in 64 out of 130 patients. Univariate analysis revealed the association between CMV reactivation and old age (46.5 vs. 39.5; P = 0.02), lower serum albumin levels at admission (2.9 vs. 3.3; P< 0.001), higher Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) (17 vs. 10; P = 0.001) at admission, and increased steroid pulse therapy (64.1% vs. 45.5%; P = 0.04) and immunosuppressive drug use (70.3% vs. 50.0%; P = 0.02) (Table 1). Log-rank tests on the above factors revealed significant differences for all except for steroid pulse therapy. Moreover, the Cox proportional hazards regression analysis on the four factors revealed the association of old age (hazard ratio [HR]: 2.03, 95% confidence interval [CI]: 1.09–3.78, P = 0.026), lower albumin (HR: 2.35, 95% CI: 1.09–5.07, P = 0.029), higher SLEDAI (HR: 1.73, 95% CI: 1.00–2.98, P = 0.048), and immunosuppressive drug use (HR: 1.94, 95% CI: 1.12–3.35, P = 0.018) with CMV reactivation. Receiver operating characteristic (ROC) curve analysis using the above four factors revealed that the sensitivity and specificity were 82.8% and 60.6%, respectively (area under the curve = 0.748, 95% CI: 0.664–0.831) for CMV reactivation when all four factors were present. The log-rank test revealed that CMV reactivation developed more frequently when two or more risk factors were present (Figure 1). Among the 64 cases with CMV reactivation, an increased number of CMV pp65 antigen-positive cells was found in the 25 cases with CMV disease than the 39 cases without the disease (6 vs. 2; P = 0.02) (Table 2). ROC curve analysis revealed that the cutoff value for organ damage development was 12 cells out of 2 slides (sensitivity: 36.0% and specificity: 94.9%).
Conclusion: Old age, low albumin levels, high disease activity, and the use of immunosuppressive drugs are possible risk factors of CMV reactivation in patients with SLE. CMV pp65 antigen-positive cells of 12 per 2 slides were determined for the diagnosis of CMV disease.
.jpg)
Table 1. Risk factors of CMV reactivation

Figure 1. Cumulative probability of CMV reactivation depending on the number of risk factors

Table 2. Comparison of patients with and without CMV disease
M. Wakatsuki: None; H. Yamashita: None; Y. Akiyama: None; S. Oyama: None; S. Aozaki: None; R. Kuwata: None; M. Yamaji: None; T. Harada: None; K. Motomura: None; Y. Nakamichi: None; H. Kaneko: None.



