Poster Session B
Sjögren’s syndrome
Session: (1365–1382) Sjögren’s Syndrome – Basic & Clinical Science Poster I
1377: Characterization of Pulmonary Manifestations of Sjögren Syndrome: A Multicenter Retrospective Study
Monday, November 13, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- LM
Loïc Meudec, MD
Université Paris-Saclay
Le Kremlin-Bicêtre, FranceDisclosure information not submitted.
Abstract Poster Presenter(s)
Loïc Meudec1, Cindy Marques2, Pierre-Antoine Juge3, Robin Dhote4, Anne-Laure Fauchais5, Emanuelle Dernis6, Olivier Vittecoq7, Alain SARAUX8, Jacques-Eric Gottenberg9, Eric Hachulla10, Véronique Le Guern11, Philippe Dieudé12, Marie-Pierre Debray13, Antoine Beurnier14, Raphaele Seror15, Xavier Mariette16 and Gaetane Nocturne17, 1CHU Kremlin-Bicêtre, Rheumatology, Le Kremlin-Bicêtre, France, 2CHU Pitié Salpétrière, Internal Medicine 1, Paris, France, 3Division of Rheumatology, Inflammation, and Immunity Brigham & Women’s Hospital, Boston, MA, 4Department of Internal Medicine, Centre Hospitalier Avicenne, Bobigny, France, 5Dupuytren Hospital, Limoges, France, 6CH Le Mans, Le Mans, France, 7CHU Rouen, Rheumatology, Rouen, France, 8CHU Brest, Brest, France, 9Rheumatology Department, Strasbourg University Hospital, Strasbourg, France, 10CHU Lille, Département de Médecine Interne et Immunologie Clinique, Centre de Référence des Maladies Auto-immunes Systémiques Rares du Nord et Nord-Ouest de France (CeRAINO), Lille, France, 11APHP Hôpital Cochin, Paris, France, 12Assistance Publique-Hôpitaux de Paris, Bichat-Claude Bernard University Hospital, INSERM UMR1152, University de Paris Cité, Department of Rheumatology, Paris, France, 13CHU Bichat, Radiology, Paris, France, 14CHU Kremlin-Bicêtre, Functional Explorations, Le Kremlin-Bicêtre, France, 15University Hospital Paris Saclay, Le Kremlin-Bicêtre, France, 16Université Paris-Saclay, Le Kremlin-Bicêtre, France, 17APHP, Le Kremlin-Bicêtre, France
Background/Purpose: Sjögren disease (Sjo) is a systemic immune-related disease with pulmonary manifestations occurring in up to 16% of patients [1], including interstitial lung disease (SS-ILD) and airway disease (SS-AD). Our objective was to assess the associated factors with SS-ILD and SS-AD and to describe these manifestations.
Methods: We performed a retrospective multicentric study, involving 9 French centers. We included Sjo patients fulfilling the ACR/EULAR 2016 criteria with a pulmonary disease evidenced by at least one clinician and one computed tomography (CT) report. We collected clinical and biological data at the visit giving access to the most exhaustive collection, pulmonary function test (PFT) and CT scans, that were all reviewed by a radiologist specialist in thoracic diseases. SS-ILD were considered progressive when associating a CT scan worsening and at least a 10% decrease of the forced vital capacity (FVC) between 2 consecutive measurements during follow-up. SS-ILD and SS-AD were compared to Sjo controls with no history of pulmonary involvement, matched on age and disease duration with a 2/1 ratio.
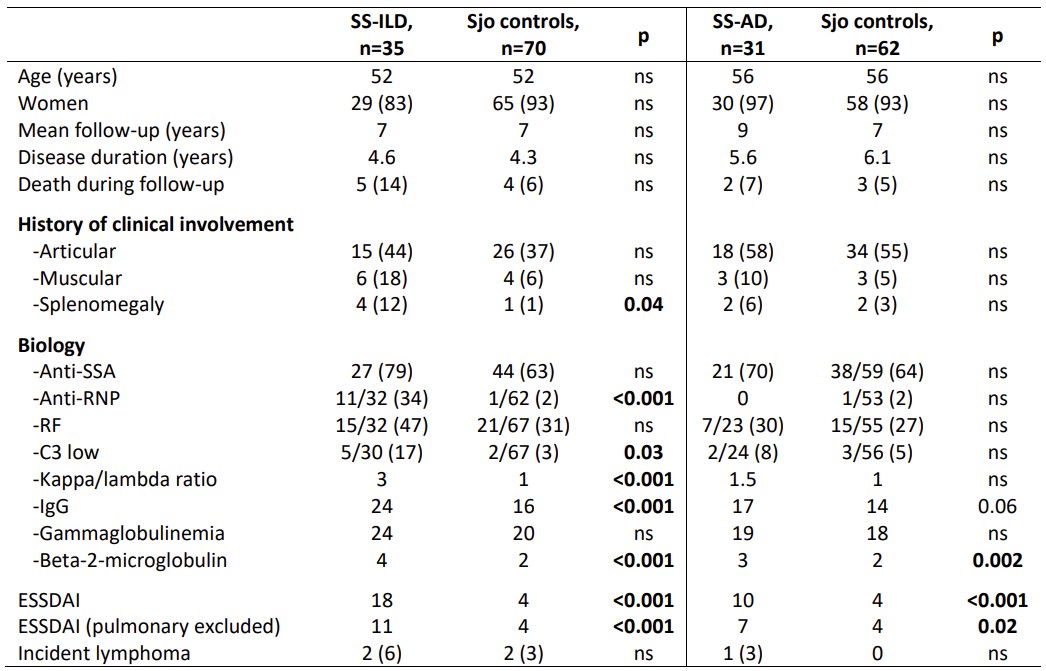
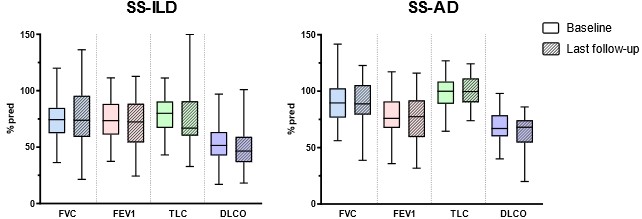
Results: We included 35 SS-ILD, 31 SS-AD and 132 Sjo controls. SS-ILD and SS-AD had significantly higher disease activity (ESSDAI) than control, even when excluding the pulmonary criteria of the score (Table 1). Thus, SS-ILD was also associated with anti-RNP antibodies and B cell biological markers at visit time. SS-ILD were mostly nonspecific interstitial pneumonia (NSIP, 26%), with fibrosis features and restrictive lung disease occurring in 46% of cases at baseline. 41% of SS-ILD were considered progressive, independently of Sjo characteristics and CT pattern. On the other hand, SS-AD were mostly diffuse, associating bronchiolitis and bronchiectasis in 60% of cases, with CT scan worsening observed in 41% of cases. Finally, both were poorly progressive in terms of PFT with respectively five and nine years of follow-up for SS-ILD and SS-AD (Figure 1).
Conclusion: SS-ILD are usually fibrosing and progressive manifestations of Sjo, associated with the disease activity and B cell biological markers. SS-AD readily associate proximal and distal airways and associate with the disease activity. Both slowly progress functionally. Bibliography 1. Ramos-Casals et al., Rheumatology. 54(12):2230‑8.
L. Meudec: None; C. Marques: None; P. Juge: None; R. Dhote: None; A. Fauchais: None; E. Dernis: AbbVie/Abbott, 2, Amgen, 2, Bristol-Myers Squibb(BMS), 2, Celgene, 2, Eli Lilly, 2, Galapagos, 2, Gilead, 2, Janssen, 2, MSD, 2, Nordic Pharma, 2, Novartis, 2, Pfizer, 2, Roche, 2, Roche-Chugai, 2, Sandoz, 2, Sanofi, 2, UCB Pharma, 2; O. Vittecoq: None; A. SARAUX: None; J. Gottenberg: AbbVie, 2, BMS, 2, 5, Galapagos, 2, Gilead, 2, Lilly, 2, MSD, 2, Novartis, 2, Pfizer, 2, 5; E. Hachulla: Bayer, 6, Boehringer-Ingelheim, 6, CSL Behring, 5, GlaxoSmithKlein(GSK), 5, 6, Johnson & Johnson, 5, 6, Roche, 5, 6, Sanofi-Genzyme, 6; V. Le Guern: None; P. Dieudé: AbbVie, 2, 6, Boehringer Ingelheim, 1, 2, 6, Bristol-Myers Squibb, 1, 2, 5, 6, Chugai, 5, Galapagos, 5, 6, Janssen, 2, 6, Novartis, 2, Pfizer, 1, 2, 5, 6; M. Debray: None; A. Beurnier: None; R. Seror: None; X. Mariette: AstraZeneca, 2, 6, BMS, 2, 6, Galapagos, 2, 6, GSK, 2, 6, Novartis, 2, 6, Pfizer, 2, 6; G. Nocturne: None.
Background/Purpose: Sjögren disease (Sjo) is a systemic immune-related disease with pulmonary manifestations occurring in up to 16% of patients [1], including interstitial lung disease (SS-ILD) and airway disease (SS-AD). Our objective was to assess the associated factors with SS-ILD and SS-AD and to describe these manifestations.
Methods: We performed a retrospective multicentric study, involving 9 French centers. We included Sjo patients fulfilling the ACR/EULAR 2016 criteria with a pulmonary disease evidenced by at least one clinician and one computed tomography (CT) report. We collected clinical and biological data at the visit giving access to the most exhaustive collection, pulmonary function test (PFT) and CT scans, that were all reviewed by a radiologist specialist in thoracic diseases. SS-ILD were considered progressive when associating a CT scan worsening and at least a 10% decrease of the forced vital capacity (FVC) between 2 consecutive measurements during follow-up. SS-ILD and SS-AD were compared to Sjo controls with no history of pulmonary involvement, matched on age and disease duration with a 2/1 ratio.
Results: We included 35 SS-ILD, 31 SS-AD and 132 Sjo controls. SS-ILD and SS-AD had significantly higher disease activity (ESSDAI) than control, even when excluding the pulmonary criteria of the score (Table 1). Thus, SS-ILD was also associated with anti-RNP antibodies and B cell biological markers at visit time. SS-ILD were mostly nonspecific interstitial pneumonia (NSIP, 26%), with fibrosis features and restrictive lung disease occurring in 46% of cases at baseline. 41% of SS-ILD were considered progressive, independently of Sjo characteristics and CT pattern. On the other hand, SS-AD were mostly diffuse, associating bronchiolitis and bronchiectasis in 60% of cases, with CT scan worsening observed in 41% of cases. Finally, both were poorly progressive in terms of PFT with respectively five and nine years of follow-up for SS-ILD and SS-AD (Figure 1).
Conclusion: SS-ILD are usually fibrosing and progressive manifestations of Sjo, associated with the disease activity and B cell biological markers. SS-AD readily associate proximal and distal airways and associate with the disease activity. Both slowly progress functionally. Bibliography 1. Ramos-Casals et al., Rheumatology. 54(12):2230‑8.

Table 1: Characteristics of SS-ILD and SS-AD compared to Sjo controls

Figure 1: PFT evolution in SS-ILD and SS-AD
L. Meudec: None; C. Marques: None; P. Juge: None; R. Dhote: None; A. Fauchais: None; E. Dernis: AbbVie/Abbott, 2, Amgen, 2, Bristol-Myers Squibb(BMS), 2, Celgene, 2, Eli Lilly, 2, Galapagos, 2, Gilead, 2, Janssen, 2, MSD, 2, Nordic Pharma, 2, Novartis, 2, Pfizer, 2, Roche, 2, Roche-Chugai, 2, Sandoz, 2, Sanofi, 2, UCB Pharma, 2; O. Vittecoq: None; A. SARAUX: None; J. Gottenberg: AbbVie, 2, BMS, 2, 5, Galapagos, 2, Gilead, 2, Lilly, 2, MSD, 2, Novartis, 2, Pfizer, 2, 5; E. Hachulla: Bayer, 6, Boehringer-Ingelheim, 6, CSL Behring, 5, GlaxoSmithKlein(GSK), 5, 6, Johnson & Johnson, 5, 6, Roche, 5, 6, Sanofi-Genzyme, 6; V. Le Guern: None; P. Dieudé: AbbVie, 2, 6, Boehringer Ingelheim, 1, 2, 6, Bristol-Myers Squibb, 1, 2, 5, 6, Chugai, 5, Galapagos, 5, 6, Janssen, 2, 6, Novartis, 2, Pfizer, 1, 2, 5, 6; M. Debray: None; A. Beurnier: None; R. Seror: None; X. Mariette: AstraZeneca, 2, 6, BMS, 2, 6, Galapagos, 2, 6, GSK, 2, 6, Novartis, 2, 6, Pfizer, 2, 6; G. Nocturne: None.



