Abstract Session
Rheumatoid arthritis (RA)
Session: Abstracts: RA – Diagnosis, Manifestations, & Outcomes I: RA-ILD (0769–0774)
0769: Development and Validation of a Combined Clinical and Genetic Risk Score for Interstitial Lung Disease in a Large, Multicenter, Prospective Rheumatoid Arthritis Cohort
Sunday, November 12, 2023
2:00 PM - 2:10 PM PT
Location: Room 6E/6D

Austin Wheeler, MD (he/him/his)
University of Nebraska Medical Center
Omaha, NE, United StatesDisclosure(s): No financial relationships with ineligible companies to disclose
Presenting Author(s)
Austin Wheeler1, Joshua Baker2, Yangyuna Yang1, Punyasha Roul1, K Wysham3, Grant Cannon4, Gary Kunkel5, Gail Kerr6, Dana Ascherman7, Paul Monach8, Andreas Reimold9, Jill Poole1, Tony Merriman10, Ted R Mikuls11 and Bryant England1, 1University of Nebraska Medical Center, Omaha, NE, 2University of Pennsylvania, Philadelphia, PA, 3VA Puget Sound/University of Washington, Seattle, WA, 4University of Utah and Salt Lake City VA, Salt Lake City, UT, 5University of Utah, Salt Lake City, UT, 6Washington DC VAMC/Georgetown and Howard Universities, Washington, DC, 7University of Pittsburgh, Pittsburgh, PA, 8VA Boston Healthcare System, Boston, MA, 9University of Texas Southwestern Medical Center, Dallas, TX, 10University of Alabama at Birmingham, Birmingham, AL, 11Division of Rheumatology and Immunology, University of Nebraska Medical Center, Omaha, NE
Background/Purpose: Rheumatoid arthritis-associated interstitial lung disease (RA-ILD) is an extra-articular manifestation of RA that causes substantial morbidity and mortality. Although some clinical and genetic risk factors (predominantly MUC5B rs35705950) have been identified, there are no routinely used tools for risk stratification. We sought to develop a risk model for ILD in a large, multicenter RA cohort.
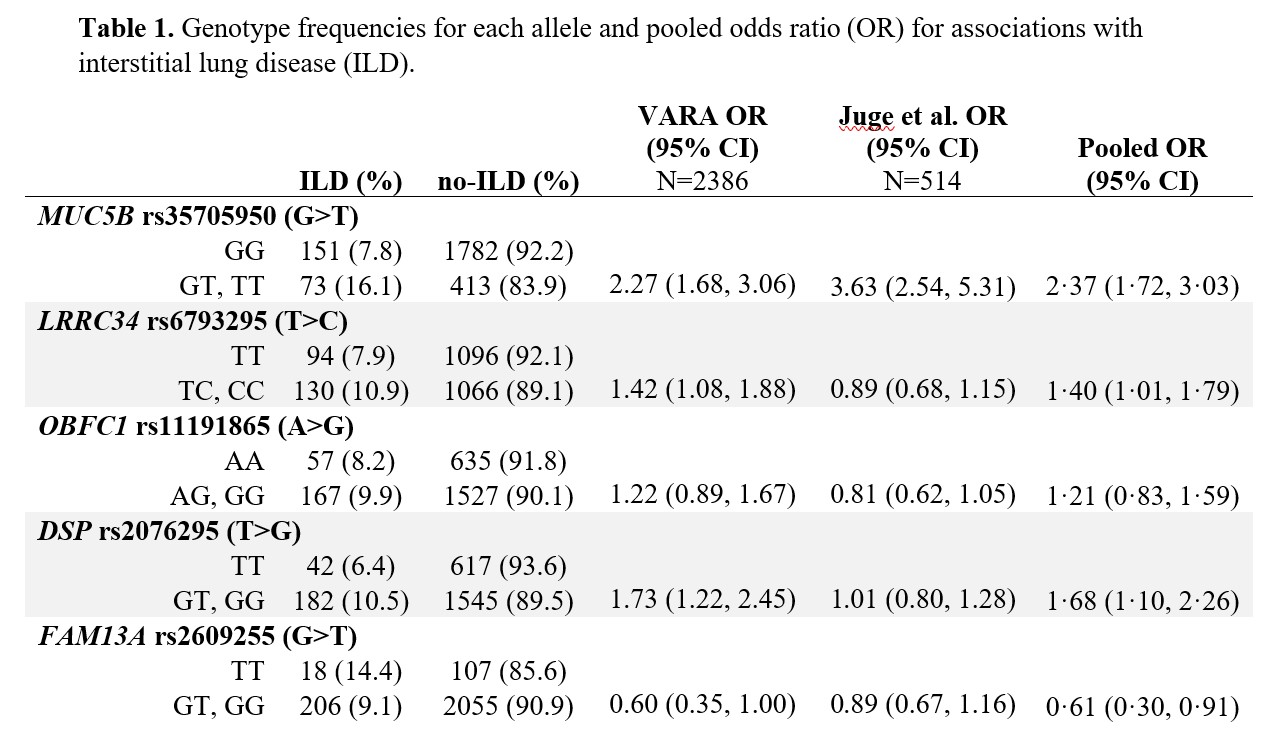
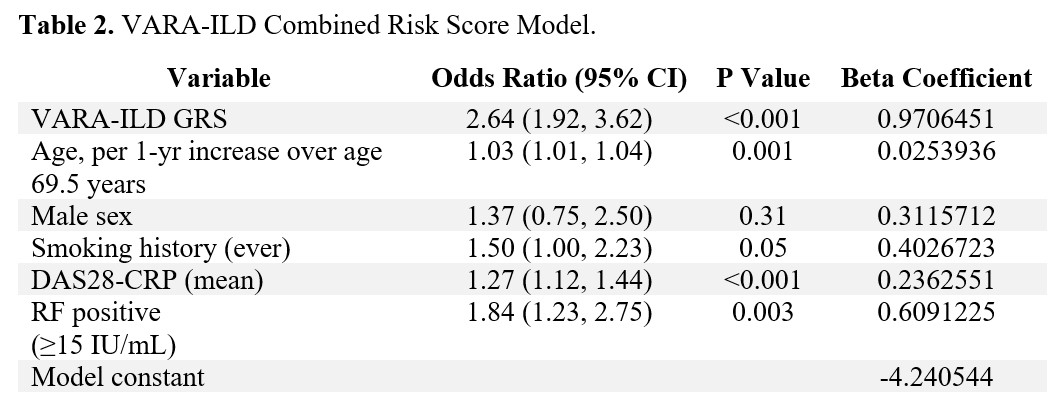
Methods: Participants were enrollees in the Veterans Affairs Rheumatoid Arthritis (VARA) registry, a multicenter prospective cohort of U.S. Veterans with RA. Participants were genotyped for single nucleotide polymorphisms (SNPs) associated with idiopathic pulmonary fibrosis, including MUC5B rs35705950 and 11 other SNPs. ILD was validated through systematic medical record review of provider diagnosis, imaging, and biopsy findings. A meta-analytic odds ratio for ILD was calculated for each SNP by pooling results from VARA (N=2386; N=224 ILD) and Juge et al. (N=514; N=272 ILD) (Table 1). A genetic risk score (GRS) was computed from variant alleles weighted by their effect size with ILD, using backwards predictor selection. The GRS was combined with established clinical risk factors (age, sex, smoking history, DAS28-CRP, and RF positivity) using logistic regression to generate the VARA-ILD Combined Risk Score. Internal validation and calibration were completed using bootstrapping techniques to address over-fitting and determine a shrinkage-corrected performance. Model performance was assessed using the area under the receiver operating curve (AUC), and models were compared via nested likelihood ratio tests as well as Akaike and Bayesian information criterion (AIC, BIC).
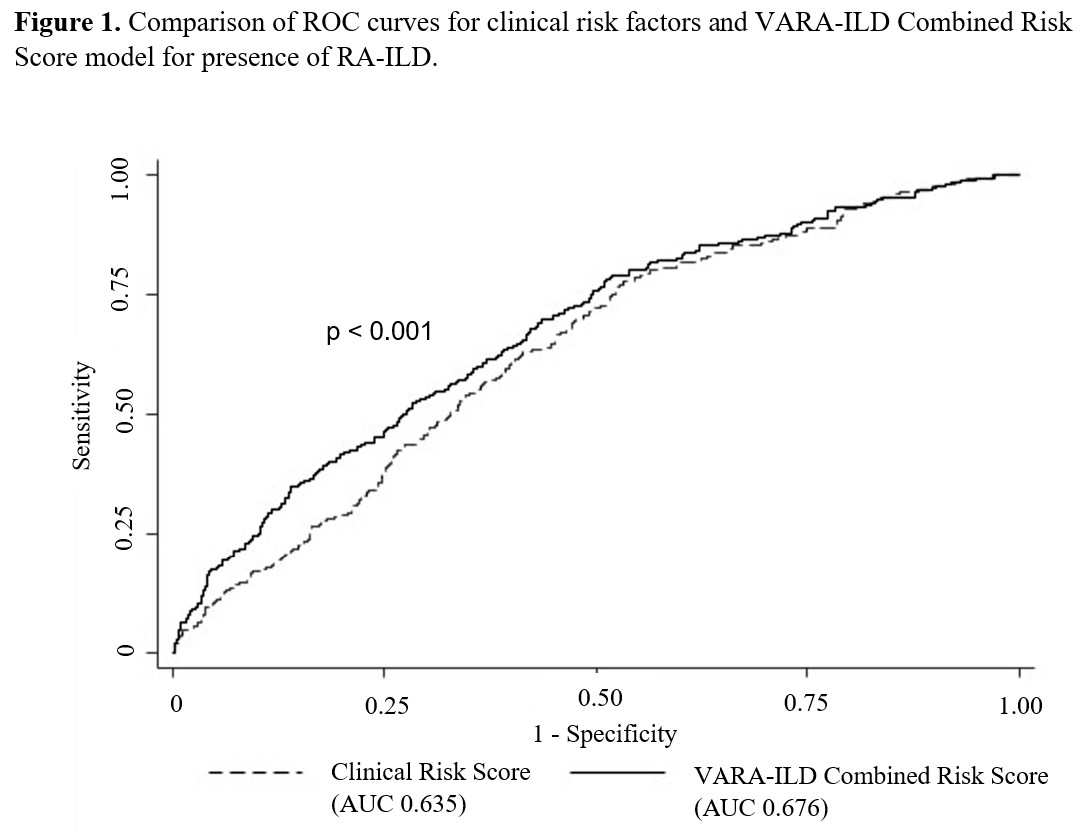
Results: Of 2,386 participants (89% male, mean age 69.5 years), 9.4% had ILD. VARA-ILD Combined Risk Score containing the GRS (based on 5 SNPs) and clinical factors (Table 2) outperformed clinical risk factors alone in discriminating ILD (AUC 0.686 vs. 0.635, p< 0.001; Figure 1). Following internal validation and bootstrapping, the shrinkage-corrected performance for combined and clinical-only models was 0.675 (95% CI 0.635, 0.709) and 0.623 (95% CI 0.584, 0.651). VARA-ILD Combined Risk Score also outperformed a model including clinical factors and the MUC5B rs35705950 promoter variant alone (AUC 0.648 [95% CI 0.612, 0.681]) based on AIC (1408 vs. 1429) and BIC (1448 vs. 1469). Demonstrating its potential role in ILD screening, utilizing a risk score cut-point of 0.05 (sensitivity 90%) would eliminate 25% (N=582) of participants from undergoing low-yield ILD diagnostic testing.
Conclusion: The VARA-ILD Combined Risk Score, including multiple genetic risk variants, discriminated ILD in a large, multicenter RA cohort better than established clinical risk factors alone. These results demonstrate the potential utility of genetic risk scores in RA-ILD identification and will support further investigation into individualized risk stratification and screening.
A. Wheeler: None; J. Baker: CorEvitas, 2, Cumberland Pharma, 2, Horizon Pharmaceuticals, 5; Y. Yang: None; P. Roul: None; K. Wysham: None; G. Cannon: None; G. Kunkel: None; G. Kerr: AstraZeneca, 2, Aurinia, 6, Horizon, 2, Janssen, 2, Pfizer, 1, Sanofi, 2; D. Ascherman: None; P. Monach: Genentech, 12, Lecture with honorarium, HI-Bio, 2; A. Reimold: None; J. Poole: AstraZeneca, 12, I have received no monies. I received anti-IL-33 monoclonal antibody from AstraZeneca for animal research sutdies.; T. Merriman: None; T. Mikuls: Elsevier, 9, Horizon Therapeutics, 2, 5, Pfizer, 2, Sanofi, 2, UCB Pharma, 2, Wolters Kluwer Health (UpToDate), 9; B. England: Boehringer-Ingelheim, 2, 5.
Background/Purpose: Rheumatoid arthritis-associated interstitial lung disease (RA-ILD) is an extra-articular manifestation of RA that causes substantial morbidity and mortality. Although some clinical and genetic risk factors (predominantly MUC5B rs35705950) have been identified, there are no routinely used tools for risk stratification. We sought to develop a risk model for ILD in a large, multicenter RA cohort.
Methods: Participants were enrollees in the Veterans Affairs Rheumatoid Arthritis (VARA) registry, a multicenter prospective cohort of U.S. Veterans with RA. Participants were genotyped for single nucleotide polymorphisms (SNPs) associated with idiopathic pulmonary fibrosis, including MUC5B rs35705950 and 11 other SNPs. ILD was validated through systematic medical record review of provider diagnosis, imaging, and biopsy findings. A meta-analytic odds ratio for ILD was calculated for each SNP by pooling results from VARA (N=2386; N=224 ILD) and Juge et al. (N=514; N=272 ILD) (Table 1). A genetic risk score (GRS) was computed from variant alleles weighted by their effect size with ILD, using backwards predictor selection. The GRS was combined with established clinical risk factors (age, sex, smoking history, DAS28-CRP, and RF positivity) using logistic regression to generate the VARA-ILD Combined Risk Score. Internal validation and calibration were completed using bootstrapping techniques to address over-fitting and determine a shrinkage-corrected performance. Model performance was assessed using the area under the receiver operating curve (AUC), and models were compared via nested likelihood ratio tests as well as Akaike and Bayesian information criterion (AIC, BIC).
Results: Of 2,386 participants (89% male, mean age 69.5 years), 9.4% had ILD. VARA-ILD Combined Risk Score containing the GRS (based on 5 SNPs) and clinical factors (Table 2) outperformed clinical risk factors alone in discriminating ILD (AUC 0.686 vs. 0.635, p< 0.001; Figure 1). Following internal validation and bootstrapping, the shrinkage-corrected performance for combined and clinical-only models was 0.675 (95% CI 0.635, 0.709) and 0.623 (95% CI 0.584, 0.651). VARA-ILD Combined Risk Score also outperformed a model including clinical factors and the MUC5B rs35705950 promoter variant alone (AUC 0.648 [95% CI 0.612, 0.681]) based on AIC (1408 vs. 1429) and BIC (1448 vs. 1469). Demonstrating its potential role in ILD screening, utilizing a risk score cut-point of 0.05 (sensitivity 90%) would eliminate 25% (N=582) of participants from undergoing low-yield ILD diagnostic testing.
Conclusion: The VARA-ILD Combined Risk Score, including multiple genetic risk variants, discriminated ILD in a large, multicenter RA cohort better than established clinical risk factors alone. These results demonstrate the potential utility of genetic risk scores in RA-ILD identification and will support further investigation into individualized risk stratification and screening.

Abbreviations: ILD (interstitial lung disease), no-ILD (no interstitial lung disease), VARA (Veterans Affairs Rheumatoid Arthritis registry), OR (odds ratio)
Pooled OR obtained by meta-analysis of OR from VARA and Juge et al. 2018, calculated via fixed-effects model.
Pooled OR obtained by meta-analysis of OR from VARA and Juge et al. 2018, calculated via fixed-effects model.

Abbreviations: interstitial lung disease (ILD), RA (rheumatoid arthritis), CI (confidence interval), DAS-28-CRP (disease activity score with 28 joints and c-reactive protein), RF (rheumatoid factor), single nucleotide polymorphism (SNP)

Abbreviations: ROC (receiver operating characteristic), ILD (interstitial lung disease), AUC (area under the curve),
P value calculated via nested likelihood ratio test.
P value calculated via nested likelihood ratio test.
A. Wheeler: None; J. Baker: CorEvitas, 2, Cumberland Pharma, 2, Horizon Pharmaceuticals, 5; Y. Yang: None; P. Roul: None; K. Wysham: None; G. Cannon: None; G. Kunkel: None; G. Kerr: AstraZeneca, 2, Aurinia, 6, Horizon, 2, Janssen, 2, Pfizer, 1, Sanofi, 2; D. Ascherman: None; P. Monach: Genentech, 12, Lecture with honorarium, HI-Bio, 2; A. Reimold: None; J. Poole: AstraZeneca, 12, I have received no monies. I received anti-IL-33 monoclonal antibody from AstraZeneca for animal research sutdies.; T. Merriman: None; T. Mikuls: Elsevier, 9, Horizon Therapeutics, 2, 5, Pfizer, 2, Sanofi, 2, UCB Pharma, 2, Wolters Kluwer Health (UpToDate), 9; B. England: Boehringer-Ingelheim, 2, 5.



