Poster Session B
Sjögren’s syndrome
Session: (1365–1382) Sjögren’s Syndrome – Basic & Clinical Science Poster I
1376: SJOGRENSER Registry: Prospective Evaluation of a Cohort of Patients with Primary Sjögren's Syndrome After 8 Years of Follow-up
Monday, November 13, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- ZP
Zulema Plaza, PhD, MSc
Fundacion Española de Reumatología
Madrid, SpainDisclosure information not submitted.
Abstract Poster Presenter(s)
Mónica Fernández-Castro1, Zulema Plaza2, Jose Rosas3, Victor Martinez-Taboada4, Alejandro Olivé5, Raúl Menor-Almagro6, SARA MANRIQUE7, Ruth López-González8, Celia Erausquin9, Antonio Naranjo9, Rocío Caño-Alameda10, Silvia Gómez-Sabater10, Jesús Alberto García Vadillo11, Belen Serrano Benavente12, Sheila Melchor13, Paula V. Estrada-Alarcón14, Angel Garcia-Aparicio15, Cristina Bohorquez16, Enrique Júdez17, Ximena Larco18, Javier Loricera19, Nerea Alcorta Lorenzo20, Beatriz Paredes Romero21, Jorge González Martín22, María Ángeles Blázquez Cañamero23, Consuelo Ramos-Giráldez24, Fernando Alonso25, Marta Domínguez26 and Jose Luis Andreu-Sánchez27, 1Rheumatology Department Hospital Puerta de Hierro Majadahonda, Majadahonda, Spain, 2Universidad Autónoma de Madrid, Madrid, Spain, 3Hospital Marina Baixa, Alicante, Spain, 4Rheumatology, Hospital Marqués de Valdecilla, Santander, Spain, 5Rheumatology Department Hospital Germans Trias i Pujol, Badalona, Spain, 6Rheumatology, Hospital Jerez, Puerto De Santa María, Spain, 7Division of Rheumatology, Hospital Regional Universitario Carlos Haya, Malaga, Spain, 8Rheumatology Department Hospital Virgen de la Concha, Zamora, Spain, 9Rheumatology Department Hospital Universitario de Gran Canaria Doctor Negrín, Las Palmas de Gran Canaria, Spain, 10Rheumatology Department, Dr. Balmis University General Hospital, Alicante. Institute for Health and Biomedical Research (ISABIAL), Alicante, Spain, 11Rheumatology Department Hospital de la Princesa, Madrid, Spain, 12Rheumatology Department Hospital Gregorio Marañón, Madrid, Spain, 13Rheumatology Department Hospital Doce de Octubre, Madrid, Spain, 14Hospital de San Juan Despí Moisès Broggi, Barcelona, Spain, 15Hospital Universitario de Toledo, Toledo, Spain, 16Rheumatology Unit, Hospital Universitario Príncipe de Asturias, Alcalá de Henares, Spain, 17Rheumatology Department Hospital de Albacete, Albacete, Spain, 18Rheumatology Department Hospital de León, León, Spain, 19Hospital Universitario Marqués de Valdecilla, Santander, Spain, 20University Hospital Donostia, San Sebastian, Spain, 21Rheumatology Department Hospital Infanta Sofía, San Sebastian de los Reyes, Spain, 22Rheumatology Department Hospital Madrid Norte Sanchinarro, Madrid, Spain, 23Rheumatology Department Hospital Ramon y Cajal, Madrid, Spain, 24Rheumatology Department Hospital Universitario Virgen de Valme, Sevilla, Spain, 25Spanish Society of Rheumatology, Madrid, Spain, 26Sociedad Española de Reumatología, Madrid, Spain, 27Rheumatology, Hospital Universitario Puerta de Hierro, Majadahonda, Spain
Background/Purpose: Describe the evolution of patients with Sjögren's syndrome (SS) in relation to the appearance of new systemic manifestations and disease activity, as well as factors associated with an unfavorable outcome.
Methods: SJÖGRENSER PROS (SS-PROS) is an observational, longitudinal, and multicenter study of patients with SS who met 2002 classification criteria under active follow-up in rheumatology clinics of 31 Spanish hospitals that participated in the cross-sectional phase of the study (SJÖGRENSER TRANS; SS-TRANS). For the prospective phase, an 8 years follow-up visit was performed (2021-2022) comparing the results with baseline visit (SS-TRANS 2013-2014, 437 patients). Medical history was reviewed and a medical interview was performed. Epidemiological, clinical and serological variables as well as causes of death were recorded. Continuous and categorical variables were analyzed using means, medians, and frequencies, with their respective deviations and interquartile ranges (Q1-Q3). Student's T test was used to establish statistical associations, considering p< 0.05 significant.
Results: Up to January 2023, 180 patients have been included, 96% women, mean age 68 years (SD 11.2) and mean evolution time from diagnosis to inclusion of 18 years (SD 7.27). Newly developed systemic involvement was: joint in 47% of patients (arthritis in 17%), hematological (cytopenia) in 44%, lung involvement in 14%, renal in 13%, digestive in 17%. %, hepatic 6%, central and peripheral nervous system (NS) 4% and 3% respectively, and cardiac 2.7%; parotid inflammation was described in 13%. In the last 8 years, 3 new lymphomas have appeared (3/180, 1.6%).
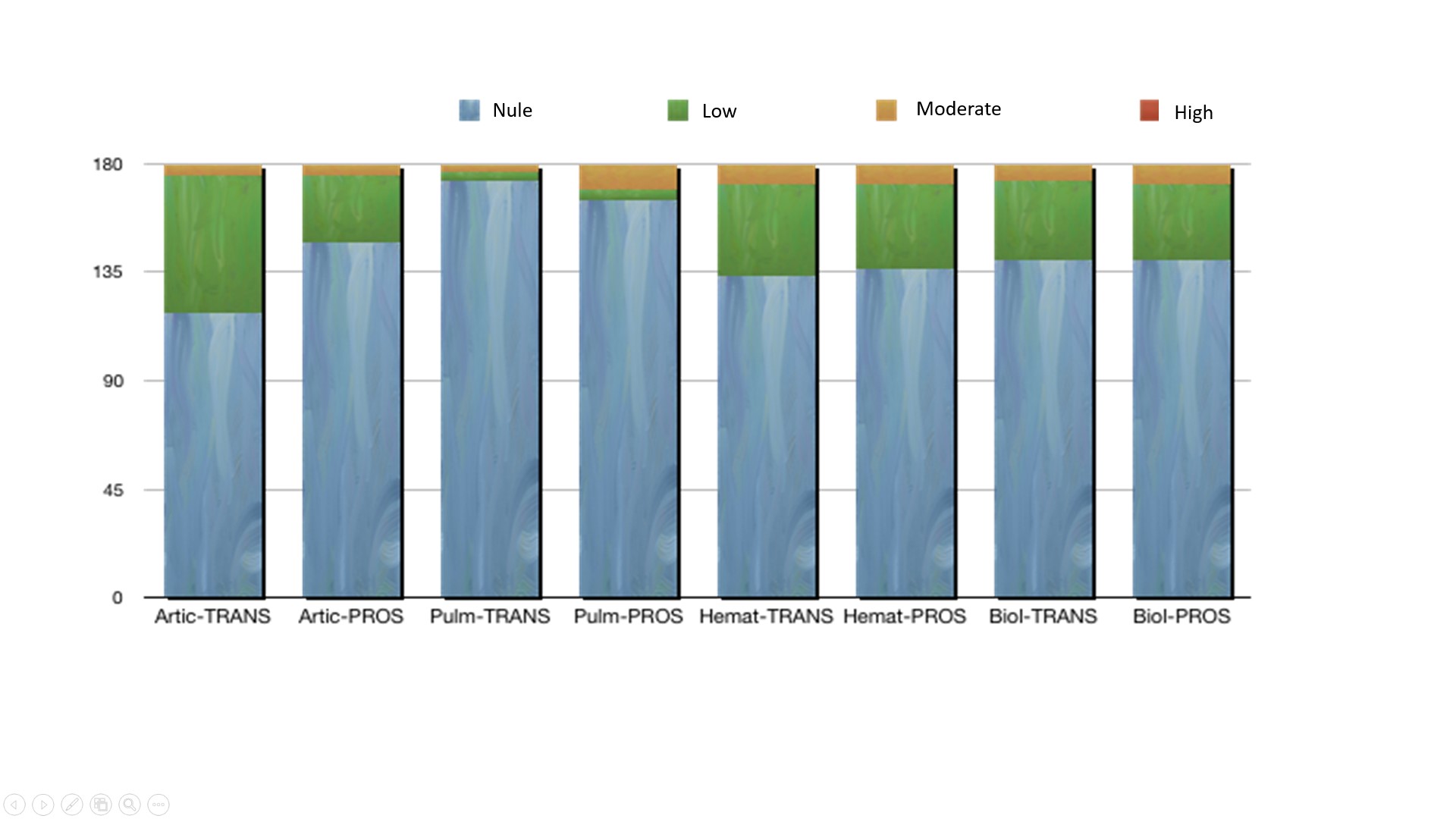
Mean of the ESSDAI in SS-PROS was lower than in SS-TRANS: 2.78 (SD 4.3; 1-4) vs. 3.66 (SD 5.15; 2-4), respectively. By domains, the greatest variations were observed in the articular, hematological and biological domains; while in the rest of the domains, stability was evidenced in ≥94% of the cases (Figure 1). By organs, the most affected organ during follow-up was the lung, with 8 patients who increased the mean value of this domain.
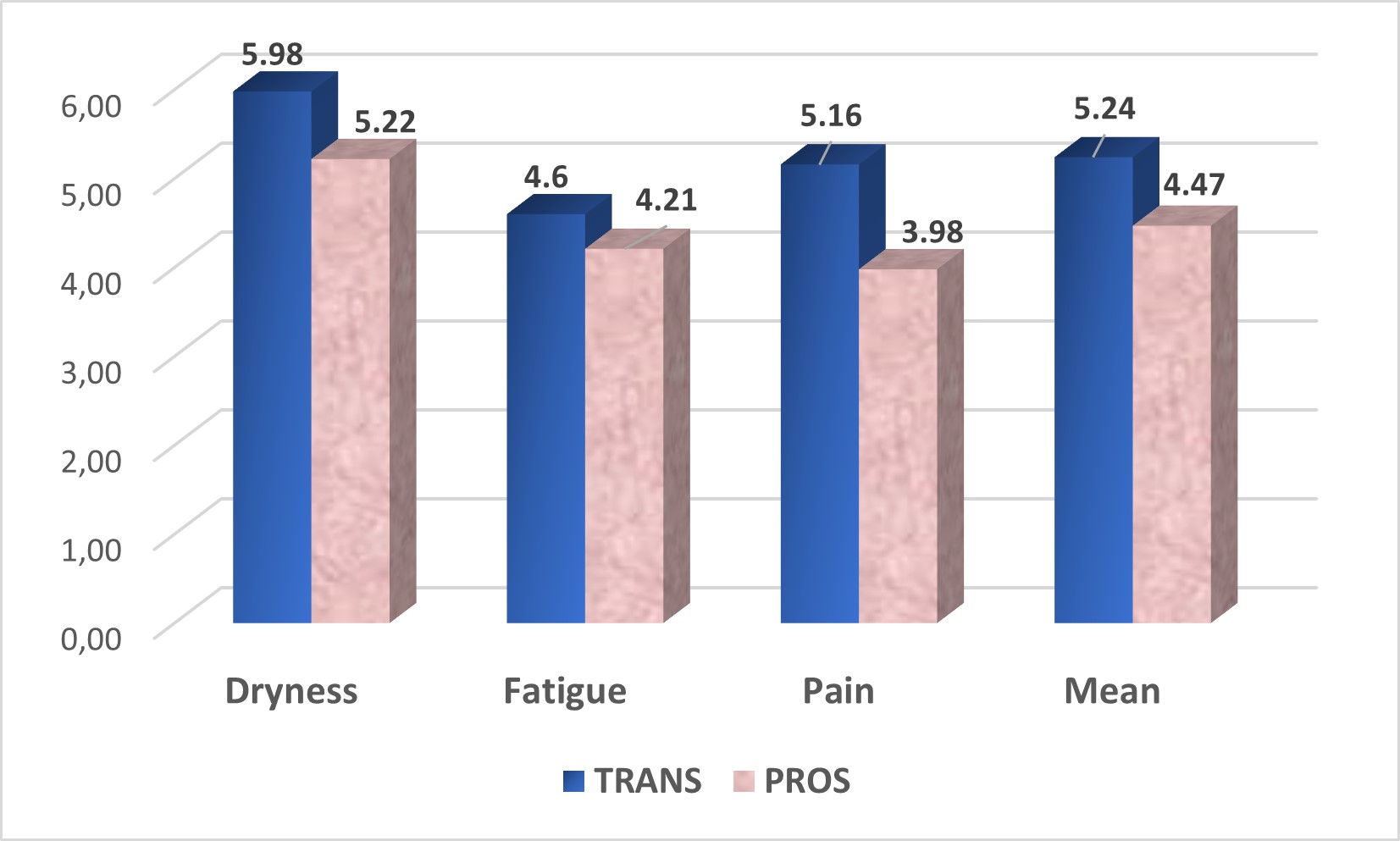
ESSPRI mean improved: 5.24 (SD 2.35; 3.67-7) vs. 4.47 (SD 2.14; 2.67-6), respectively; being greater in the VAS of pain, 5.16 (SD 3.02; 3- 8) vs. 3.98 (SD 2.95; 1- 7), respectively (Figure 2).
Twenty-seven patients out of 180 have died since their inclusion in SS-TRANS (15%), 89% women, with a mean age of 75 years (SD 11.5; 65-84.5).
Comparing baseline data visit (SS-TRANS) from the group of deceased (during SS-PROS) and of non-deceased (remaining under follow-up in SS-PROS), we observed that age (70 years; SD 11; 59-77), disease evolution time (11.5 years; SD 8; 4-17) and ESSDAI (5.22; SD 6.35; 0-8), were higher in the deceased vs. non-deceased (age 59 years, SD 12, p< 0.001; evolution time 9 years, SD7, p=0.078; ESSDAI 3.38, SD 4.88, 0-4, p0.087). There were no differences in ESSPRI.
Conclusion: Patients with SS develop new systemic manifestations over the years, despite maintaining or improving ESSDAI, suggesting the need of a close follow-up. The ESSPRI varies little over time, which represents a great challenge for the scientific community. Mortality in this cohort is 15%. Greater age, time of evolution and baseline ESSDAI were associated with a worse outcome.
M. Fernández-Castro: None; Z. Plaza: None; J. Rosas: None; V. Martinez-Taboada: None; A. Olivé: None; R. Menor-Almagro: None; S. MANRIQUE: None; R. López-González: None; C. Erausquin: AbbVie/Abbott, 12, financial support to assist meetings; A. Naranjo: None; R. Caño-Alameda: None; S. Gómez-Sabater: None; J. García Vadillo: None; B. Serrano Benavente: None; S. Melchor: None; P. Estrada-Alarcón: None; A. Garcia-Aparicio: None; C. Bohorquez: None; E. Júdez: None; X. Larco: None; J. Loricera: None; N. Alcorta Lorenzo: None; B. Paredes Romero: None; J. González Martín: None; M. Blázquez Cañamero: Gedeon Richter, 6, GlaxoSmithKlein(GSK), 6, UCB, 6; C. Ramos-Giráldez: None; F. Alonso: None; M. Domínguez: None; J. Andreu-Sánchez: None.
Background/Purpose: Describe the evolution of patients with Sjögren's syndrome (SS) in relation to the appearance of new systemic manifestations and disease activity, as well as factors associated with an unfavorable outcome.
Methods: SJÖGRENSER PROS (SS-PROS) is an observational, longitudinal, and multicenter study of patients with SS who met 2002 classification criteria under active follow-up in rheumatology clinics of 31 Spanish hospitals that participated in the cross-sectional phase of the study (SJÖGRENSER TRANS; SS-TRANS). For the prospective phase, an 8 years follow-up visit was performed (2021-2022) comparing the results with baseline visit (SS-TRANS 2013-2014, 437 patients). Medical history was reviewed and a medical interview was performed. Epidemiological, clinical and serological variables as well as causes of death were recorded. Continuous and categorical variables were analyzed using means, medians, and frequencies, with their respective deviations and interquartile ranges (Q1-Q3). Student's T test was used to establish statistical associations, considering p< 0.05 significant.
Results: Up to January 2023, 180 patients have been included, 96% women, mean age 68 years (SD 11.2) and mean evolution time from diagnosis to inclusion of 18 years (SD 7.27). Newly developed systemic involvement was: joint in 47% of patients (arthritis in 17%), hematological (cytopenia) in 44%, lung involvement in 14%, renal in 13%, digestive in 17%. %, hepatic 6%, central and peripheral nervous system (NS) 4% and 3% respectively, and cardiac 2.7%; parotid inflammation was described in 13%. In the last 8 years, 3 new lymphomas have appeared (3/180, 1.6%).
Mean of the ESSDAI in SS-PROS was lower than in SS-TRANS: 2.78 (SD 4.3; 1-4) vs. 3.66 (SD 5.15; 2-4), respectively. By domains, the greatest variations were observed in the articular, hematological and biological domains; while in the rest of the domains, stability was evidenced in ≥94% of the cases (Figure 1). By organs, the most affected organ during follow-up was the lung, with 8 patients who increased the mean value of this domain.
ESSPRI mean improved: 5.24 (SD 2.35; 3.67-7) vs. 4.47 (SD 2.14; 2.67-6), respectively; being greater in the VAS of pain, 5.16 (SD 3.02; 3- 8) vs. 3.98 (SD 2.95; 1- 7), respectively (Figure 2).
Twenty-seven patients out of 180 have died since their inclusion in SS-TRANS (15%), 89% women, with a mean age of 75 years (SD 11.5; 65-84.5).
Comparing baseline data visit (SS-TRANS) from the group of deceased (during SS-PROS) and of non-deceased (remaining under follow-up in SS-PROS), we observed that age (70 years; SD 11; 59-77), disease evolution time (11.5 years; SD 8; 4-17) and ESSDAI (5.22; SD 6.35; 0-8), were higher in the deceased vs. non-deceased (age 59 years, SD 12, p< 0.001; evolution time 9 years, SD7, p=0.078; ESSDAI 3.38, SD 4.88, 0-4, p0.087). There were no differences in ESSPRI.
Conclusion: Patients with SS develop new systemic manifestations over the years, despite maintaining or improving ESSDAI, suggesting the need of a close follow-up. The ESSPRI varies little over time, which represents a great challenge for the scientific community. Mortality in this cohort is 15%. Greater age, time of evolution and baseline ESSDAI were associated with a worse outcome.

Figure 1: ESSDAI analysis by domains comparing SjögrenSER TRANS vs. SjögrenSER PROS

Figure 2: Mean ESSPRI in SjögrenSER TRANS compare with SjögrenSER PROS
M. Fernández-Castro: None; Z. Plaza: None; J. Rosas: None; V. Martinez-Taboada: None; A. Olivé: None; R. Menor-Almagro: None; S. MANRIQUE: None; R. López-González: None; C. Erausquin: AbbVie/Abbott, 12, financial support to assist meetings; A. Naranjo: None; R. Caño-Alameda: None; S. Gómez-Sabater: None; J. García Vadillo: None; B. Serrano Benavente: None; S. Melchor: None; P. Estrada-Alarcón: None; A. Garcia-Aparicio: None; C. Bohorquez: None; E. Júdez: None; X. Larco: None; J. Loricera: None; N. Alcorta Lorenzo: None; B. Paredes Romero: None; J. González Martín: None; M. Blázquez Cañamero: Gedeon Richter, 6, GlaxoSmithKlein(GSK), 6, UCB, 6; C. Ramos-Giráldez: None; F. Alonso: None; M. Domínguez: None; J. Andreu-Sánchez: None.



