Poster Session A
Infection-related rheumatic syndromes
Session: (0196–0228) Infection-related Rheumatic Disease Poster
0227: Associations of DMARDs with Post-Acute Sequelae of COVID-19 in Patients with Systemic Autoimmune Rheumatic Diseases: A Prospective Study
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- RV
Rathnam Venkat, BS
Tufts University School of Medicine
Boston, MA, United StatesDisclosure information not submitted.
Abstract Poster Presenter(s)
Rathnam Venkat1, Xiaosong Wang2, Naomi Patel3, Yumeko Kawano2, Abigail Schiff2, Emily Kowalski2, Claire Cook3, Kathleen Vanni2, Grace Qian2, Katarina Bade4, Alene Saavedra2, Shruthi Srivatsan3, Zachary Williams3, Zachary Wallace5 and Jeffrey Sparks6, 1Tufts University School of Medicine, Boston, MA, 2Brigham and Women's Hospital, Boston, MA, 3Massachusetts General Hospital, Boston, MA, 4Brigham and Women's Hospital, Boston, MA, 5Massachusetts General Hospital, Newton, MA, 6Division of Rheumatology, Inflammation, and Immunity, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA
Background/Purpose: Post-acute sequelae of COVID-19 (PASC, or "long COVID") is defined by the CDC as COVID-19 symptoms persisting for ≥28 days after infection. Patients with systemic autoimmune rheumatic diseases (SARDs) may be at higher risk for PASC due to their underlying disease and immunosuppressive medications. Disease-modifying antirheumatic drugs (DMARDs), particularly CD20 inhibitors, have been associated with severe COVID-19, but the effect of DMARD use on PASC risk is unclear. Therefore, we investigated the association of baseline DMARD use and PASC among patients with SARDs.
Methods: We invited all SARD patients with COVID-19 within a large healthcare system to participate in a prospective study. Participants completed a survey ≥28 days after confirmed COVID-19 infection, and we analyzed surveys completed from 3/11/2021 to 5/5/2023. The survey collected data on demographics, SARD characteristics, COVID-19 vaccination status, DMARD use at COVID-19 diagnosis, and COVID-19 symptoms and disease course. We categorized DMARD classes by mechanism of action; those taking combination DMARDs were classified by hierarchy of targeted therapy. PASC was defined by any symptom associated with COVID-19 that persisted for ≥28 days. We used logistic regression to estimate odds ratios (OR) for PASC by DMARD class, adjusting for potential confounders.
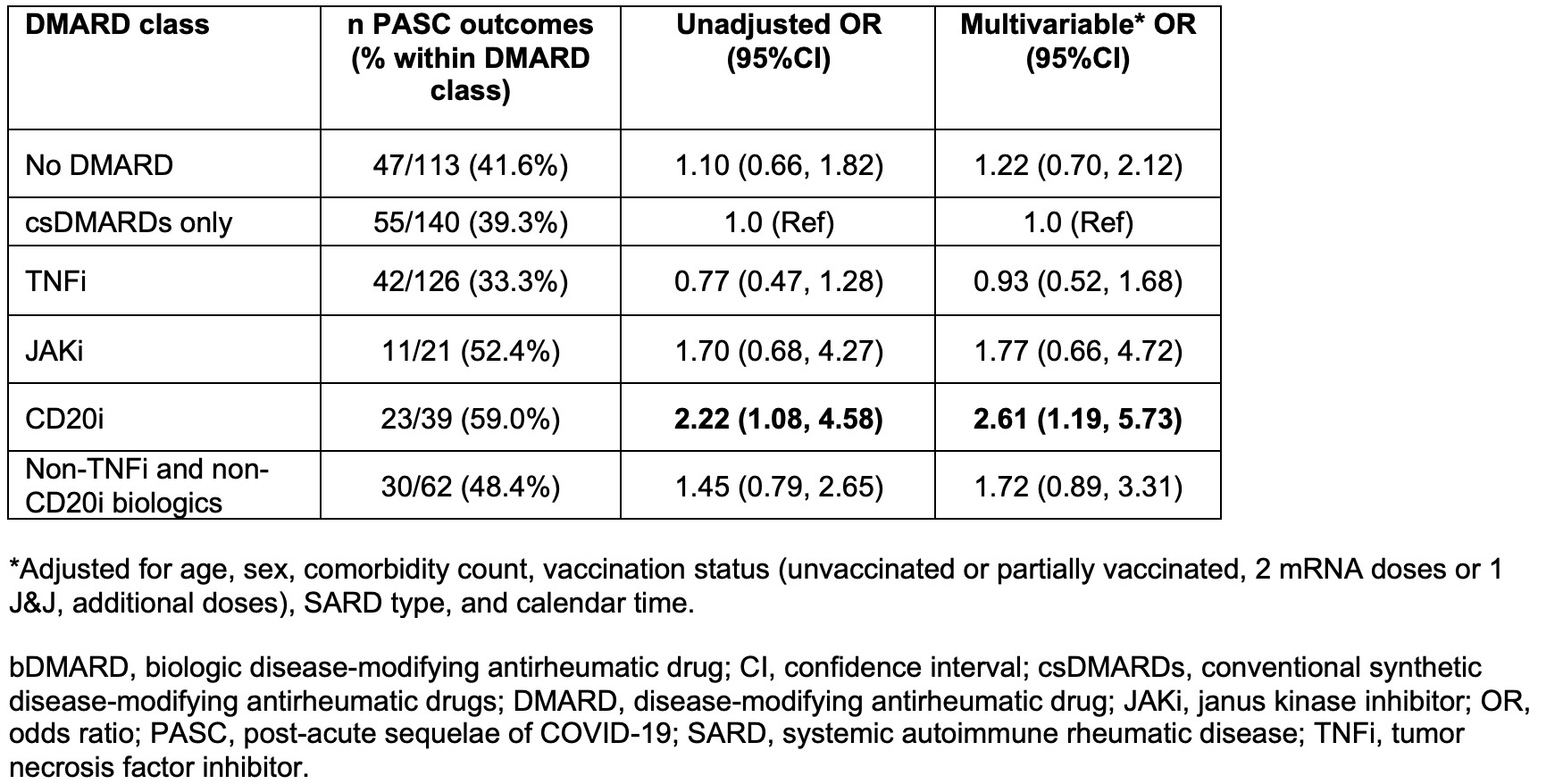
Results: We analyzed 501 patients with SARDs and COVID-19 (mean age 52.7 years, 80.2% female), of which 208 (42%) had PASC. The most common SARD type was inflammatory arthritis (53.7%), followed by connective tissue disease (21.6%). Participants with PASC were more likely to be female (88.0% vs. 74.7%, p=0.0002), less likely to have had additional COVID-19 vaccine doses beyond the primary series (45.2% vs. 55.0%, p=0.031), and more likely to be infected with pre-Omicron variants (53.4% vs. 37.5%, p=0.0004) compared to participants without PASC. Participants with PASC were also less likely to be on TNF inhibitors (20.2% vs. 28.7%, p=0.031) and more likely to be on CD20 inhibitors (11.1% vs. 5.5%, p=0.021). Participants with PASC were more likely to have been hospitalized for COVID-19 (13.9% vs. 4.1%, p< 0.0001). There were no statistically significant differences in age, race, comorbidity count, SARD type, and other DMARD classes, when comparing those with and without PASC (Table 1). After adjusting for comorbidity count, vaccination status, SARD type, and calendar time of infection, SARD patients using CD20 inhibitors had an OR for PASC of 2.61 (95%CI 1.19-5.73) compared to those on conventional synthetic DMARDs (Table 2).
Conclusion: In this prospective study, SARD patients on CD20 inhibitors at COVID-19 onset had increased risk for PASC. This analysis extends previous studies linking CD20 inhibitors with acute COVID-19 severity and suggests vigilance is needed to prevent COVID-19 and monitor for PASC in this vulnerable population. Mechanisms linking B cell depletion with PASC risk may include persistent infection, dysregulated immune response following acute infection, and acute COVID-19 severity from impaired humoral immunity.
.jpg)
R. Venkat: None; X. Wang: None; N. Patel: Arrivo Bio, 2, Chronius Health, 2, FVC Health, 2; Y. Kawano: None; A. Schiff: None; E. Kowalski: None; C. Cook: None; K. Vanni: None; G. Qian: None; K. Bade: None; A. Saavedra: None; S. Srivatsan: None; Z. Williams: None; Z. Wallace: BioCryst, 2, Bristol-Myers Squibb(BMS), 5, Horizon, 1, 2, 5, MedPace, 2, Novartis, 1, PPD, 2, Sanofi, 1, 5, Shionogi, 1, Visterra, 1, 2, Zenas, 1, 2; J. Sparks: AbbVie, 2, Amgen, 2, Boehringer Ingelheim, 2, Bristol-Myers Squibb, 2, 5, Gilead, 2, Inova Diagnostics, 2, Janssen, 2, Optum, 2, Pfizer, 2, ReCor, 2.
Background/Purpose: Post-acute sequelae of COVID-19 (PASC, or "long COVID") is defined by the CDC as COVID-19 symptoms persisting for ≥28 days after infection. Patients with systemic autoimmune rheumatic diseases (SARDs) may be at higher risk for PASC due to their underlying disease and immunosuppressive medications. Disease-modifying antirheumatic drugs (DMARDs), particularly CD20 inhibitors, have been associated with severe COVID-19, but the effect of DMARD use on PASC risk is unclear. Therefore, we investigated the association of baseline DMARD use and PASC among patients with SARDs.
Methods: We invited all SARD patients with COVID-19 within a large healthcare system to participate in a prospective study. Participants completed a survey ≥28 days after confirmed COVID-19 infection, and we analyzed surveys completed from 3/11/2021 to 5/5/2023. The survey collected data on demographics, SARD characteristics, COVID-19 vaccination status, DMARD use at COVID-19 diagnosis, and COVID-19 symptoms and disease course. We categorized DMARD classes by mechanism of action; those taking combination DMARDs were classified by hierarchy of targeted therapy. PASC was defined by any symptom associated with COVID-19 that persisted for ≥28 days. We used logistic regression to estimate odds ratios (OR) for PASC by DMARD class, adjusting for potential confounders.
Results: We analyzed 501 patients with SARDs and COVID-19 (mean age 52.7 years, 80.2% female), of which 208 (42%) had PASC. The most common SARD type was inflammatory arthritis (53.7%), followed by connective tissue disease (21.6%). Participants with PASC were more likely to be female (88.0% vs. 74.7%, p=0.0002), less likely to have had additional COVID-19 vaccine doses beyond the primary series (45.2% vs. 55.0%, p=0.031), and more likely to be infected with pre-Omicron variants (53.4% vs. 37.5%, p=0.0004) compared to participants without PASC. Participants with PASC were also less likely to be on TNF inhibitors (20.2% vs. 28.7%, p=0.031) and more likely to be on CD20 inhibitors (11.1% vs. 5.5%, p=0.021). Participants with PASC were more likely to have been hospitalized for COVID-19 (13.9% vs. 4.1%, p< 0.0001). There were no statistically significant differences in age, race, comorbidity count, SARD type, and other DMARD classes, when comparing those with and without PASC (Table 1). After adjusting for comorbidity count, vaccination status, SARD type, and calendar time of infection, SARD patients using CD20 inhibitors had an OR for PASC of 2.61 (95%CI 1.19-5.73) compared to those on conventional synthetic DMARDs (Table 2).
Conclusion: In this prospective study, SARD patients on CD20 inhibitors at COVID-19 onset had increased risk for PASC. This analysis extends previous studies linking CD20 inhibitors with acute COVID-19 severity and suggests vigilance is needed to prevent COVID-19 and monitor for PASC in this vulnerable population. Mechanisms linking B cell depletion with PASC risk may include persistent infection, dysregulated immune response following acute infection, and acute COVID-19 severity from impaired humoral immunity.
.jpg)
Table 1. Baseline characteristics at time of COVID-19 diagnosis according to PASC status among patients with systemic autoimmune rheumatic diseases (n=501).

Table 2. Associations of baseline use of disease-modifying antirheumatic drugs with PASC risk (n=501).
R. Venkat: None; X. Wang: None; N. Patel: Arrivo Bio, 2, Chronius Health, 2, FVC Health, 2; Y. Kawano: None; A. Schiff: None; E. Kowalski: None; C. Cook: None; K. Vanni: None; G. Qian: None; K. Bade: None; A. Saavedra: None; S. Srivatsan: None; Z. Williams: None; Z. Wallace: BioCryst, 2, Bristol-Myers Squibb(BMS), 5, Horizon, 1, 2, 5, MedPace, 2, Novartis, 1, PPD, 2, Sanofi, 1, 5, Shionogi, 1, Visterra, 1, 2, Zenas, 1, 2; J. Sparks: AbbVie, 2, Amgen, 2, Boehringer Ingelheim, 2, Bristol-Myers Squibb, 2, 5, Gilead, 2, Inova Diagnostics, 2, Janssen, 2, Optum, 2, Pfizer, 2, ReCor, 2.



