Poster Session B
Systemic lupus erythematosus (SLE)
Session: (0899–0933) SLE – Etiology & Pathogenesis Poster
0907: Epstein Barr Virus Reactivation May Associate with Transition from Incomplete to Systemic Lupus Erythematosus
Monday, November 13, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- BJ
Benjamin Jones, BS (he/him/his)
Oklahoma State University
Oklahoma City, OK, United StatesDisclosure information not submitted.
Abstract Poster Presenter(s)
Benjamin Jones1, Carla Guthridge1, Marci Beel1, Catriona Wagner2, Stan Kamp1, Cristina Arriens3, Joan Merrill1, Teresa Aberle1, Nancy Redinger1, Rebecca Wood4, Lauren Guthridge1, Susan Macwana1, Wade DeJager1, Joel Guthridge1 and Judith James1, 1Oklahoma Medical Research Foundation, Oklahoma City, OK, 2Oklahoma Medical Research Foundation, Santa Cruz, CA, 3Oklahoma Medical Research Foundation and University of Oklahoma Health Sciences Center, Department of Arthritis & Clinical Immunology, Oklahoma City, OK, 4University of Oklahoma Health Sciences Center, Edmond, OK
Background/Purpose: Patients with incomplete lupus erythematosus (ILE) have evidence of SLE but do not fulfill SLE classification criteria. Although most ILE patients maintain a relatively mild disease course, about 20% will transition to SLE. However, the factors influencing SLE development in subsets of ILE patients are largely unknown. We previously found that serological markers of EBV reactivation, measured by antibodies against EBV-early antigen (EBV-EA), were associated with increased numbers of SLE-associated autoantibodies and eventual SLE transition in SLE relatives. In addition, EBV reactivation is associated with increased disease activity in SLE patients. Therefore, this study determined whether reduced EBV reactivation contributes to milder disease course in ILE and whether increased EBV reactivation may influence transition to SLE in ILE patients.
Methods: Serum samples consented for use in these studies were obtained from existing collections in the Arthritis & Clinical Immunology Biorepository at the Oklahoma Medical Research Foundation. Healthy controls (n=72) were ANA-negative with no ACR 1997 criteria. ILE/ILE subjects (n=286) met less than 4 ACR criteria and were categorized as ILE by SLICC criteria. ILE/SLE subjects (n=148) met less than 4 ACR criteria but met SLE classification by SLICC criteria. SLE subjects (n=173) were classified as SLE by both ACR and SLICC criteria. Subjects were matched by age, sex, and ancestry. Serum autoantibodies against dsDNA, chromatin, SSA/Ro, SSB/La, Sm, SmRNP, RNP, and centromere-B were determined by BioPlex 2200® ANA kit. Serum antibodies against EBV-Viral Capsid Antigen (VCA), EBV-EA, CMV, and HSV-1 were determined by ELISA.
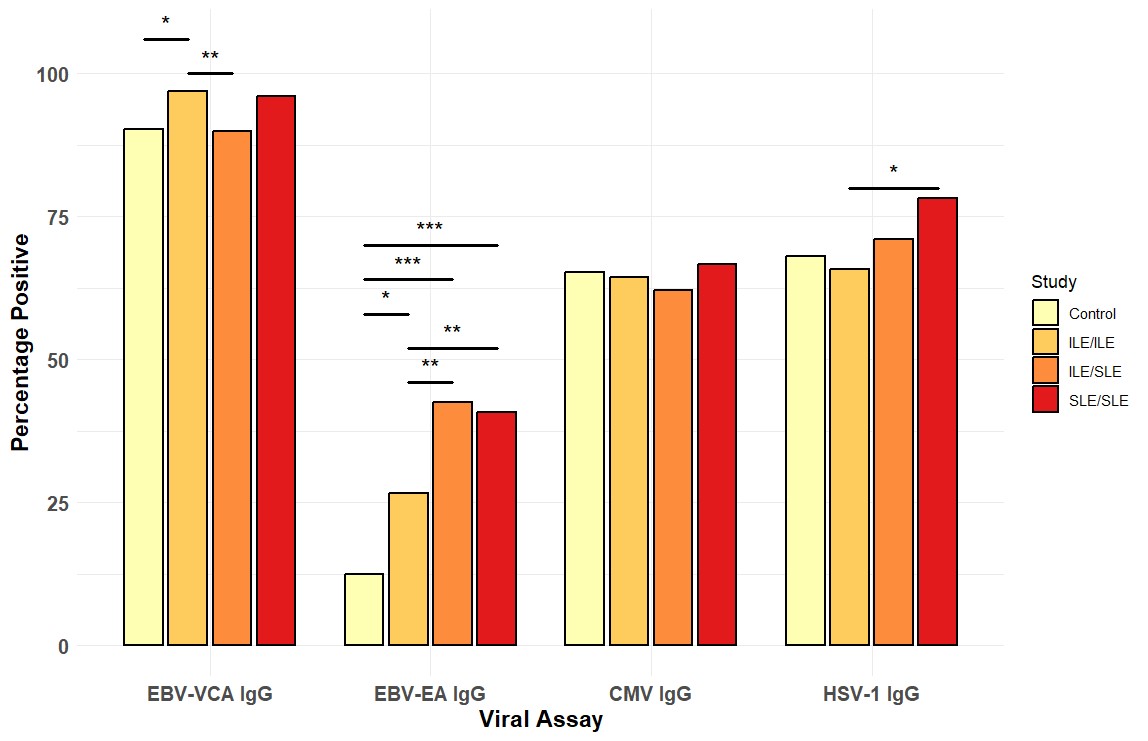
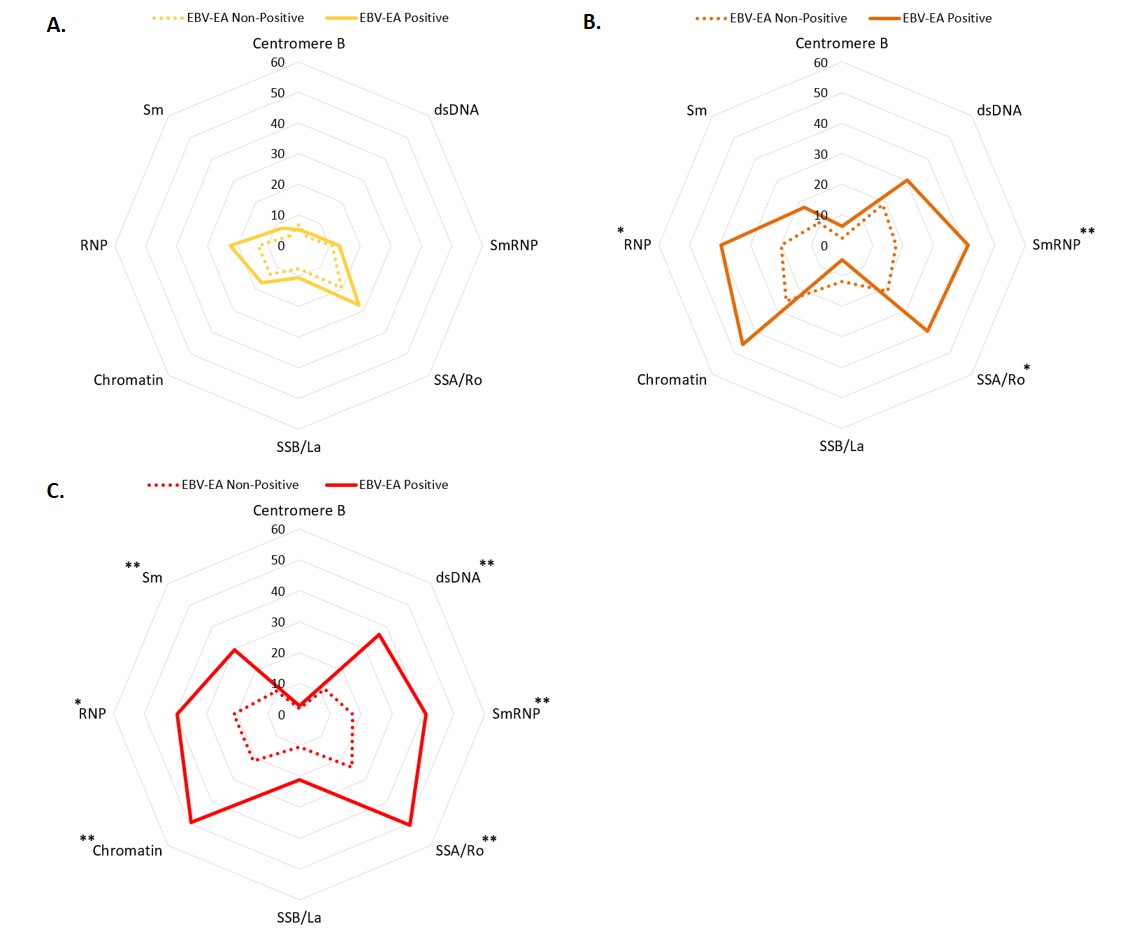
Results: EBV-EA positivity was more prevalent in ILE/ILE, ILE/SLE, and SLE patients compared to controls (Figure 1). However, EBV-EA positivity was less prevalent in ILE/ILE compared to ILE/SLE and SLE patients (Figure 1). EBV-VCA positivity was more prevalent in ILE/ILE compared to controls or ILE/SLE, but neither ILE/SLE nor SLE significantly differed from controls, consistent with previous studies (Figure 1). In contrast, CMV and HSV-1 positivity did not differ between the groups, except for a slight increase in SLE patients compared to ILE/ILE patients (Figure 1). In ILE/ILE patients, SLE-associated autoantibodies were not associated with EBV-EA positivity (Figure 2A). However, EBV-EA positivity was associated with anti-SmRNP, -SSA/Ro, and -RNP autoantibodies in ILE/SLE patients (Figure 2B). In addition to these autoantibodies, EBV-EA positivity also associated with anti-dsDNA, -chromatin, and -Sm in SLE patients (Figure 2C).
Conclusion: Serological markers of EBV reactivation are lower in ILE/ILE patients compared to ILE/SLE and SLE patients, suggesting that reduced EBV reactivation may contribute to the milder disease course observed in ILE patients. In addition, EBV-EA positivity was associated with SLE-associated autoantibodies in ILE/SLE patients but not ILE/ILE patients. EBV-EA positivity correlated with positivity for a higher number of SLE-associated autoantibodies in SLE patients compared to those with ILE/SLE. Therefore, increased EBV reactivation may be a harbinger of disease transition in ILE patients.
B. Jones: None; C. Guthridge: None; M. Beel: None; C. Wagner: None; S. Kamp: None; C. Arriens: AstraZeneca, 1, 5, 6, Aurinia, 6, Bristol-Myers Squibb, 1, 5, Cabaletta, 1, GSK, 1, Kezar, 1, UCB, 1; J. Merrill: AbbVie, 2, Alexion, 2, Alumis, 2, Amgen, 2, AstraZeneca, 2, 5, Aurinia, 2, Bristol Myers Squibb, 2, 5, EMD Serono, 2, Genentech, 2, Gilead, 2, GlaxoSmithKline, 2, 5, Lilly, 2, Merck, 2, Pfizer, 2, Provention, 2, Remegen, 2, Sanofi, 2, UCB Pharma, 2, Zenas, 2; T. Aberle: None; N. Redinger: None; R. Wood: None; L. Guthridge: None; S. Macwana: None; W. DeJager: None; J. Guthridge: None; J. James: Bristol-Myers Squibb(BMS), 5, GlaxoSmithKlein(GSK), 2, Novartis, 2, Progentec Biosciences, 5.
Background/Purpose: Patients with incomplete lupus erythematosus (ILE) have evidence of SLE but do not fulfill SLE classification criteria. Although most ILE patients maintain a relatively mild disease course, about 20% will transition to SLE. However, the factors influencing SLE development in subsets of ILE patients are largely unknown. We previously found that serological markers of EBV reactivation, measured by antibodies against EBV-early antigen (EBV-EA), were associated with increased numbers of SLE-associated autoantibodies and eventual SLE transition in SLE relatives. In addition, EBV reactivation is associated with increased disease activity in SLE patients. Therefore, this study determined whether reduced EBV reactivation contributes to milder disease course in ILE and whether increased EBV reactivation may influence transition to SLE in ILE patients.
Methods: Serum samples consented for use in these studies were obtained from existing collections in the Arthritis & Clinical Immunology Biorepository at the Oklahoma Medical Research Foundation. Healthy controls (n=72) were ANA-negative with no ACR 1997 criteria. ILE/ILE subjects (n=286) met less than 4 ACR criteria and were categorized as ILE by SLICC criteria. ILE/SLE subjects (n=148) met less than 4 ACR criteria but met SLE classification by SLICC criteria. SLE subjects (n=173) were classified as SLE by both ACR and SLICC criteria. Subjects were matched by age, sex, and ancestry. Serum autoantibodies against dsDNA, chromatin, SSA/Ro, SSB/La, Sm, SmRNP, RNP, and centromere-B were determined by BioPlex 2200® ANA kit. Serum antibodies against EBV-Viral Capsid Antigen (VCA), EBV-EA, CMV, and HSV-1 were determined by ELISA.
Results: EBV-EA positivity was more prevalent in ILE/ILE, ILE/SLE, and SLE patients compared to controls (Figure 1). However, EBV-EA positivity was less prevalent in ILE/ILE compared to ILE/SLE and SLE patients (Figure 1). EBV-VCA positivity was more prevalent in ILE/ILE compared to controls or ILE/SLE, but neither ILE/SLE nor SLE significantly differed from controls, consistent with previous studies (Figure 1). In contrast, CMV and HSV-1 positivity did not differ between the groups, except for a slight increase in SLE patients compared to ILE/ILE patients (Figure 1). In ILE/ILE patients, SLE-associated autoantibodies were not associated with EBV-EA positivity (Figure 2A). However, EBV-EA positivity was associated with anti-SmRNP, -SSA/Ro, and -RNP autoantibodies in ILE/SLE patients (Figure 2B). In addition to these autoantibodies, EBV-EA positivity also associated with anti-dsDNA, -chromatin, and -Sm in SLE patients (Figure 2C).
Conclusion: Serological markers of EBV reactivation are lower in ILE/ILE patients compared to ILE/SLE and SLE patients, suggesting that reduced EBV reactivation may contribute to the milder disease course observed in ILE patients. In addition, EBV-EA positivity was associated with SLE-associated autoantibodies in ILE/SLE patients but not ILE/ILE patients. EBV-EA positivity correlated with positivity for a higher number of SLE-associated autoantibodies in SLE patients compared to those with ILE/SLE. Therefore, increased EBV reactivation may be a harbinger of disease transition in ILE patients.

Percentage of positive viral assay results in controls (n=72), ILE/ILE (n=286), ILE/SLE (n=148), and SLE (n=173). * p < 0.05, **p < 0.01, *** p < 0.001 by Fisher’s exact test with a Benjamini-Hochberg correction.

Percentage of positive autoantibody results according to EBV-EA result in (A) ILE/ILE (n=286) (EBV-EA Positive n = 176, Non-Positive n = 210), (B) ILE/SLE (n=148) (EBV-EA Positive n = 63, Non-Positive n = 85), and (C) SLE (n=173) (EBV-EA Positive n = 71, Non-Positive n = 102). * p < 0.05, **p < 0.01, *** p < 0.001 by Fisher’s exact test with a Benjamini-Hochberg correction.
B. Jones: None; C. Guthridge: None; M. Beel: None; C. Wagner: None; S. Kamp: None; C. Arriens: AstraZeneca, 1, 5, 6, Aurinia, 6, Bristol-Myers Squibb, 1, 5, Cabaletta, 1, GSK, 1, Kezar, 1, UCB, 1; J. Merrill: AbbVie, 2, Alexion, 2, Alumis, 2, Amgen, 2, AstraZeneca, 2, 5, Aurinia, 2, Bristol Myers Squibb, 2, 5, EMD Serono, 2, Genentech, 2, Gilead, 2, GlaxoSmithKline, 2, 5, Lilly, 2, Merck, 2, Pfizer, 2, Provention, 2, Remegen, 2, Sanofi, 2, UCB Pharma, 2, Zenas, 2; T. Aberle: None; N. Redinger: None; R. Wood: None; L. Guthridge: None; S. Macwana: None; W. DeJager: None; J. Guthridge: None; J. James: Bristol-Myers Squibb(BMS), 5, GlaxoSmithKlein(GSK), 2, Novartis, 2, Progentec Biosciences, 5.



