Poster Session A
Epidemiology, health policy and outcomes
Session: (0117–0144) Epidemiology & Public Health Poster I
0131: Identifying Antinuclear Antibody Positive Individuals at Risk for Systemic Autoimmune Disease: Development and Validation of a Real-Time Risk Model
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
.png)
April Barnado, MD, MSc
Vanderbilt University Medical Center
Nashville, TN, United StatesDisclosure(s): No financial relationships with ineligible companies to disclose
Abstract Poster Presenter(s)
April Barnado, Ryan Moore, Hank Domenico, Sarah Green, Alex Camai, Ashley Suh, Bryan Han, Katherine Walker, Audrey Anderson, Lannawill Caruth, Anish Katta, Allison McCoy and Daniel W. Byrne, Vanderbilt University Medical Center, Nashville, TN
Background/Purpose: Up to 20% of the general population has a positive ANA without having autoimmune disease. Currently, no tools exist to help clinicians interpret the significance of a positive ANA. We developed and validated a risk model that uses readily available data in the electronic health record (EHR) to identify positive ANA individuals at risk for developing systemic autoimmune disease.
Methods: Using a large de-identified EHR, we randomly selected 2000 individuals with a positive ANA (titer ≥ 1:80) to perform chart review to determine if they were diagnosed with a systemic autoimmune disease by a rheumatologist. Non-rheumatic conditions such as autoimmune thyroiditis and autoimmune hepatitis were not counted as cases. Patients with prior diagnoses of systemic autoimmune disease at time of first positive ANA were excluded. A priori, we selected variables for the risk model including demographics, billing codes, and labs, previously identified in lupus risk models. We performed logistic regression using the following predictors: age at time of first positive ANA, sex, ANA titer, presence of another autoantibody (i.e. dsDNA), platelet count, and billing codes. A random selection of individuals was set aside for model validation. We assessed model performance in the training and validation sets using c-statistic and calibration curves.
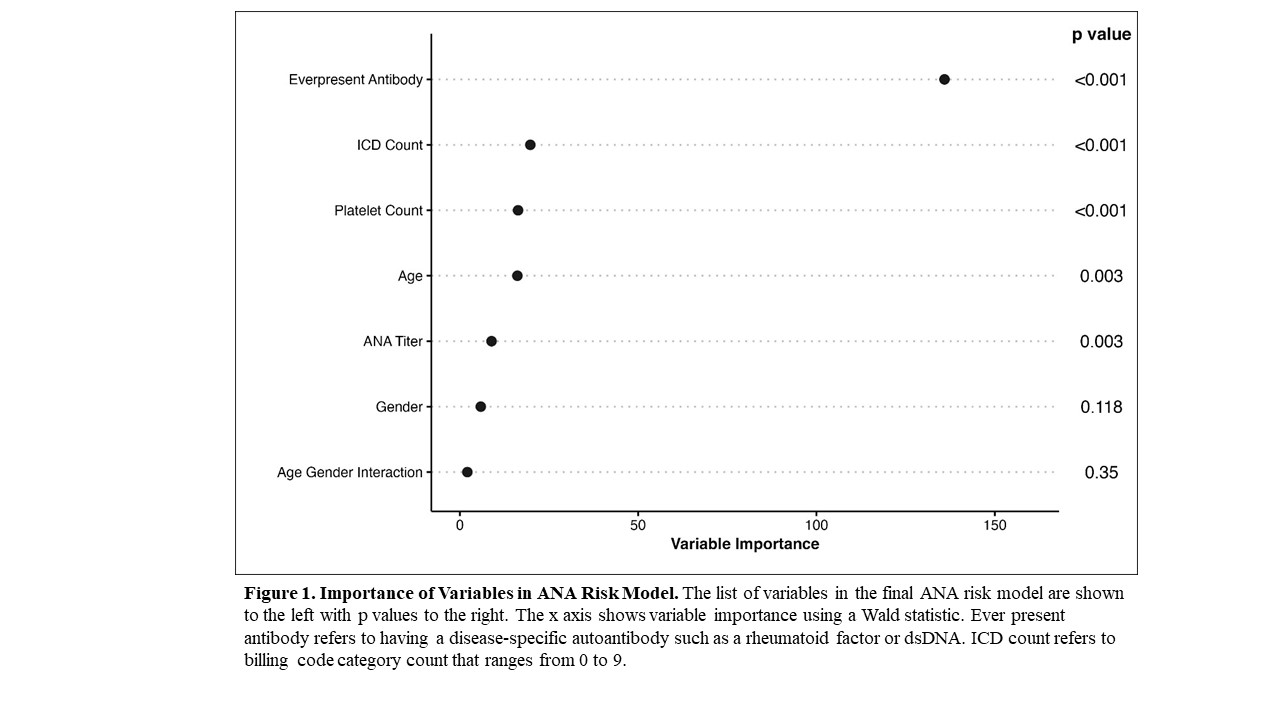
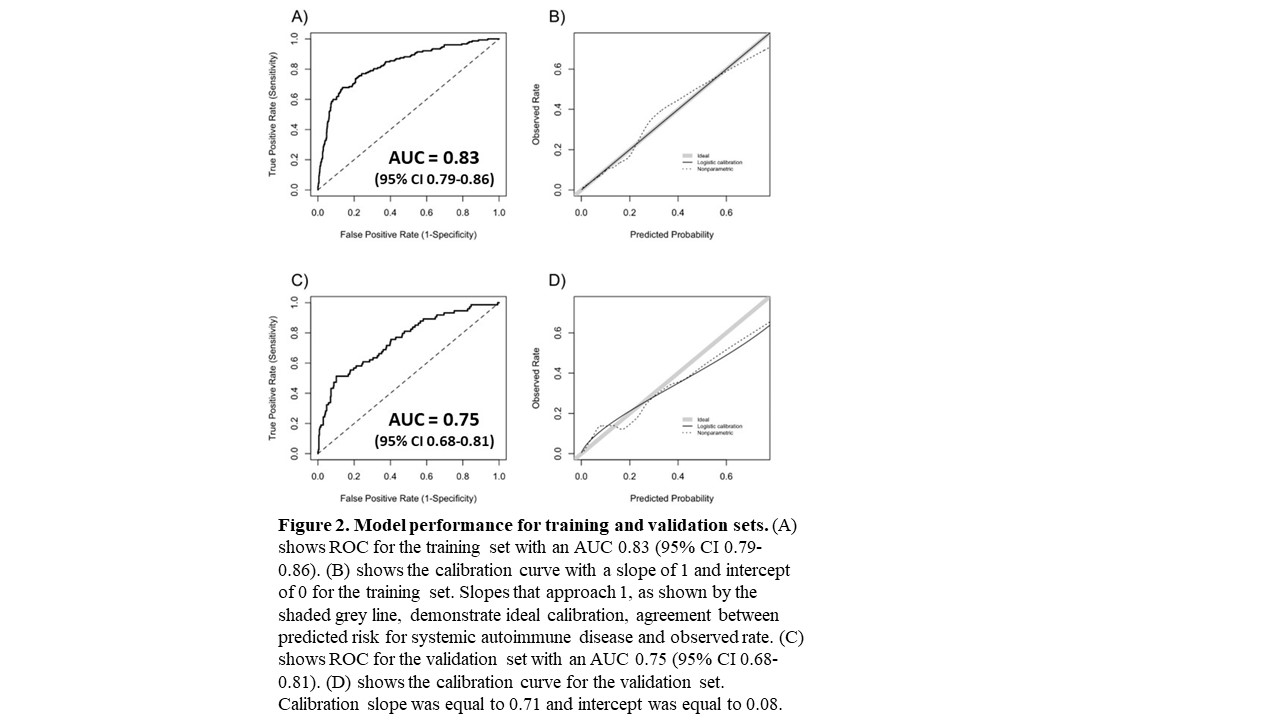
Results: We assembled training (n = 1030) and validation (n = 449) sets with 15% (n = 152) and 16% (n = 74) having systemic autoimmune disease, respectively. The most frequent systemic autoimmune diseases in the training set were SLE at 18% (n = 28), other at 16% (n = 24), undifferentiated connective tissue disease at 16% (n = 24), and rheumatoid arthritis at 15% (n = 22). Other consisted of psoriatic arthritis/plaque psoriasis and inflammatory bowel disease. Individuals with systemic autoimmune diseases were younger (41.8 ± 21.5 vs. 47.9 ± 19.3 years, p = 0.003), more likely to be female (84% vs. 70%, p < 0.001), have a higher ANA titer (≥1:160 vs. 1:80) (90% vs. 79%, p = 0.002), higher platelet count (274 ± 113 vs. 229 ± 96 K/uL, p < 0.001), more likely to have a disease-specific autoantibody (51% vs. 9%, p < 0.001), and a higher count of the nine billing code categories (scale 0 to 9) compared to individuals without disease (0.9 ± 0.9 vs. 0.6 ± 0.8, p < 0.001) (Table 1). No significant differences were found in race, ethnicity, or white blood cell count. The most important variables in the model included having a disease-specific autoantibody, billing code count, and platelet count (Figure 1). For the training set, model AUC was 0.83 (95% CI 0.79-0.86) with good calibration (Figure 2). For the validation set, model AUC was 0.75 (95% CI 0.68-0.81).
Conclusion: We developed and validated a risk model for systemic autoimmune disease in positive ANA individuals. The model is important because it utilizes readily available EHR data and helps risk stratify positive ANA individuals. In the setting of a national shortage of rheumatologists and frequent referrals for positive ANAs, a risk stratifying tool for positive ANA individuals is critical. High-risk individuals could be evaluated urgently to prevent delays in diagnosis while low-risk individuals could be reassured.
.jpg)
A. Barnado: None; R. Moore: None; H. Domenico: None; S. Green: None; A. Camai: None; A. Suh: None; B. Han: None; K. Walker: None; A. Anderson: None; L. Caruth: None; A. Katta: None; A. McCoy: None; D. Byrne: None.
Background/Purpose: Up to 20% of the general population has a positive ANA without having autoimmune disease. Currently, no tools exist to help clinicians interpret the significance of a positive ANA. We developed and validated a risk model that uses readily available data in the electronic health record (EHR) to identify positive ANA individuals at risk for developing systemic autoimmune disease.
Methods: Using a large de-identified EHR, we randomly selected 2000 individuals with a positive ANA (titer ≥ 1:80) to perform chart review to determine if they were diagnosed with a systemic autoimmune disease by a rheumatologist. Non-rheumatic conditions such as autoimmune thyroiditis and autoimmune hepatitis were not counted as cases. Patients with prior diagnoses of systemic autoimmune disease at time of first positive ANA were excluded. A priori, we selected variables for the risk model including demographics, billing codes, and labs, previously identified in lupus risk models. We performed logistic regression using the following predictors: age at time of first positive ANA, sex, ANA titer, presence of another autoantibody (i.e. dsDNA), platelet count, and billing codes. A random selection of individuals was set aside for model validation. We assessed model performance in the training and validation sets using c-statistic and calibration curves.
Results: We assembled training (n = 1030) and validation (n = 449) sets with 15% (n = 152) and 16% (n = 74) having systemic autoimmune disease, respectively. The most frequent systemic autoimmune diseases in the training set were SLE at 18% (n = 28), other at 16% (n = 24), undifferentiated connective tissue disease at 16% (n = 24), and rheumatoid arthritis at 15% (n = 22). Other consisted of psoriatic arthritis/plaque psoriasis and inflammatory bowel disease. Individuals with systemic autoimmune diseases were younger (41.8 ± 21.5 vs. 47.9 ± 19.3 years, p = 0.003), more likely to be female (84% vs. 70%, p < 0.001), have a higher ANA titer (≥1:160 vs. 1:80) (90% vs. 79%, p = 0.002), higher platelet count (274 ± 113 vs. 229 ± 96 K/uL, p < 0.001), more likely to have a disease-specific autoantibody (51% vs. 9%, p < 0.001), and a higher count of the nine billing code categories (scale 0 to 9) compared to individuals without disease (0.9 ± 0.9 vs. 0.6 ± 0.8, p < 0.001) (Table 1). No significant differences were found in race, ethnicity, or white blood cell count. The most important variables in the model included having a disease-specific autoantibody, billing code count, and platelet count (Figure 1). For the training set, model AUC was 0.83 (95% CI 0.79-0.86) with good calibration (Figure 2). For the validation set, model AUC was 0.75 (95% CI 0.68-0.81).
Conclusion: We developed and validated a risk model for systemic autoimmune disease in positive ANA individuals. The model is important because it utilizes readily available EHR data and helps risk stratify positive ANA individuals. In the setting of a national shortage of rheumatologists and frequent referrals for positive ANAs, a risk stratifying tool for positive ANA individuals is critical. High-risk individuals could be evaluated urgently to prevent delays in diagnosis while low-risk individuals could be reassured.
.jpg)


A. Barnado: None; R. Moore: None; H. Domenico: None; S. Green: None; A. Camai: None; A. Suh: None; B. Han: None; K. Walker: None; A. Anderson: None; L. Caruth: None; A. Katta: None; A. McCoy: None; D. Byrne: None.



