Poster Session A
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (0483–0509) Spondyloarthritis Including Psoriatic Arthritis – Diagnosis, Manifestations, & Outcomes Poster I: PsA
0495: Prevalence of Undiagnosed Inflammatory Bowel Disease in Patients with Spondyloarthritis: EISER Study
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- ZP
Zulema Plaza, PhD, MSc
Fundacion Española de Reumatología
Madrid, SpainDisclosure information not submitted.
Abstract Poster Presenter(s)
Jesus Sanz1, Zulema Plaza2, Jordi Gratacos Masmitja3, Iago Rodríguez -Lago4, Elisa Trujillo5, Ignacio Marin-Jimenez6, Eva Perez-Pampin7, Manuel Barreiro de Acosta8, Antonio Aznar Esquivel9, Marta Carrillo Palau10, Maria Luz Garcia Vivar11, Maria Carmen Muñoz12, Lourdes Ladehesa Pineda13, Eva Iglesias Flores14, carolina Merino15, Yago gonzalez-Lama16, Marta Arévalo Salaet17, Xavier calvet18, Anahy Maria Brandy-Garcia19, Marta Izquierdo Romero20, SARA MANRIQUE21, Raúl Olmedo22, Jose Francisco Garcia Llorente23, Sandra Pérez24, Inmaculada Ros25, Nuria Rull26, Jose Antonio Pinto Tasende27, Patricia Ucha Abal28, Carlos M González29, Fernando José Rodríguez Martínez30, Soledad Serrano Ladron de Guevara31, Marta Domínguez32, Francisco Javier Prado33, Enrique González-Dávila34 and Ana Gutierrez-Casbas35, 1Hospital Universitario Puerta de Hierro, Majadahonda, Spain, 2Universidad Autónoma de Madrid, Madrid, Spain, 3University Hospital Parc Taulí, Sabadell, Spain, 4Gastroenterology department Hospital Galdakao, Galdakao, Spain, 5Hospital Universitario de Canarias, Santa Cruz de Tenerife, Spain, 6Public Health System, Madrid, Spain, 7Rheumatology Department Complejo Hospitalario Universitario Santiago, Santiago de compostela, Spain, 8Gastroenterology department Complejo Hospitalario Universitario Santiago, Santiago de compostela, Spain, 9Rheumatology Department Hospital Universitario Canarias, Santa Cruz de Tenerife, Spain, 10Gastroenterology department Hospital Universitario Canarias, Santa Cruz de Tenerife, Spain, 11Basurto University Hospital, Bilbao, Spain, 12Gastroenterology department Basurto Hospital, Bilbao, Spain, 13Rheumatology Department Reina Sofia Universitary Hospital, Cordoba, Spain, 14Gastroenterology department Hospital Reina Sofia, Cordoba, Spain, 15Rheumatology Department Hospital Universitario Puerta de Hierro, Majadahonda (Madrid), Spain, 16Gastroenterology department Hospital Puerta de Hierro Majadahonda, Majadahonda, Spain, 17Rheumatology Department University Hospital Parc Taulí, Sabadell, Spain, 18Gastroenterology department University Hospital Parc Taulí, Sabadell, Spain, 19Hospital Germans Trias i Pujol, Badalona, Spain, 20Gastroenterology department Hospital Cabueñes, Gijon, Spain, 21Division of Rheumatology, Hospital Regional Universitario Carlos Haya, Malaga, Spain, 22Gastroenterology department Hospital Regional Universitario Málaga, Malaga, Spain, 23Hospital de Gadakao Usansolo Osakidetza, Bilbao, Spain, 24Gastroenterology department Hospital Galdakao, Galdakao, Spain, 25Rheumatology Department HUSLL, Palma de Mallorca, Spain, 26Gastroenterology department HUSLL, Palma de Mallorca, Spain, 27Rheumatology department, Complexo Hospitalario Universitario A Coruña (CHUAC). Instituto de Investigación Biomédica A Coruña (INIBIC), A Coruña, Spain, 28Gastroenterology department Complejo Hospitalario Universitario A Coruña, A Coruña, Spain, 29Rheumatology Department Hospital Gregorio Marañón, Instituto de Investigación Sanitaria Gregorio Marañón, Madrid, Spain, 30Rheumatology Department Hospital Universitario General Santa Lucia, Murcia, Spain, 31Gastroenterology department Hospital Universitario General Santa Lucia, Murcia, Spain, 32Sociedad Española de Reumatología, Madrid, Spain, 33Research department Hospital Infantil de México Federico Gómez, Mexico City, Mexico, 34Department of Mathematics, Statistics and Operation Reseach La Laguna University, La Laguna, Spain, 35Gastroenterology department Hospital General Universitario de. Dr. Balmis de Alicante Servicio Digestivo. ISABIAL y CIBERehd, Alicante, Spain
Background/Purpose: EISER is a cross-sectional, multicenter, observational, SER-GETECCU cooperative study involving 13 Spanish hospitals whose main objective was to estimate the prevalence of undiagnosed Inflammatory Bowel Disease (IBD) in patients with spondyloarthritis (SpA), including axial (axSpA) (radiographic axSpA-r and non-radiographic; axSpA-nr) and psoriatic arthritis (PsA).
Methods: We selected patients ≥18 years of age from the NHS Rheumatology Department, SpA diagnosed according to CASPAR criteria for PsA and ASAS for axSpA. Patients under treatment with biologics were excluded. Patients currently treated with systemic steroids or in the previous 30 days were not included. Patients over 50 years of age were included if a colonoscopy wasn't performed in the last three years or, if a colonoscopy was performed but did not meet the criteria of valid colonoscopy.
Patients were recruited by the rheumatologist who collected demographic, clinical and treatment data. A fecal calprotectin (FC) determination was performed using the Quantum Blue rapid test. FC was assessed and all patients with a FC ≥80 µg/g underwent an endoscopic study by the gastroenterologist. Patients in whom the result of the endoscopic study was normal underwent to an endoscopic capsule study or magnetic resonance imaging.
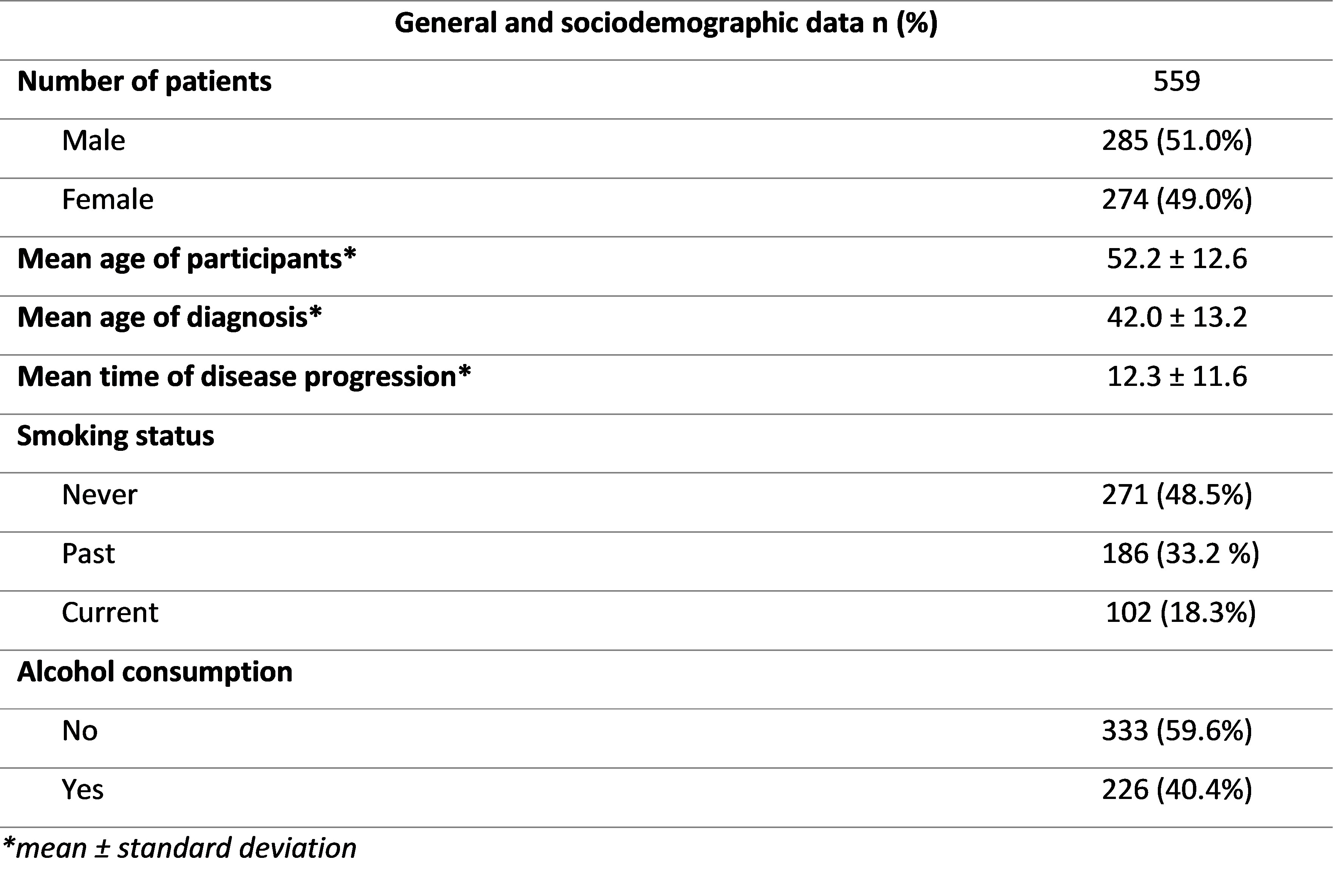
Results: A total of 559 patients were included, 51.0% of whom were men. The mean age of the participants was 52.2 years, with a mean age at diagnosis of 42 years. Mean time of disease evolution was 12.3 years (Table 1).
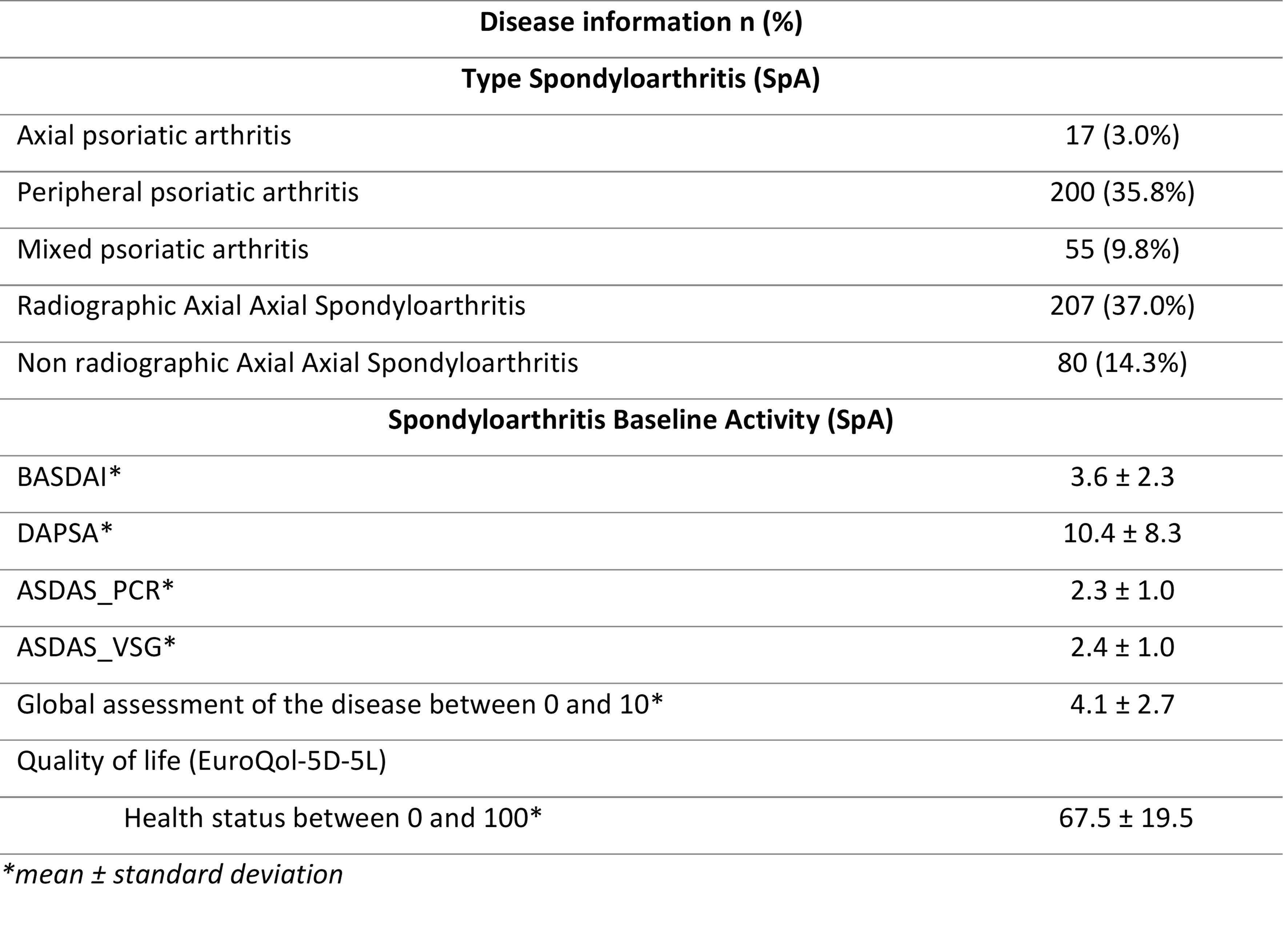
Regarding the type of SpA, the most frequent form was axSpA-r (37.0%), followed by peripheral PsA (35.8%; Table 2). Concerning disease activity data in axSpA BASDAI 3.6, ASDAS_PCR 2.3, ASDAS_VSG 2.4 and of patients with PsA DAPSA of 10.4 (Table 2). Global assessment and mean health status reported by patients was 4.1 (0 to 10 scale) and 67.5 (0 to 100 scale; Table 2), respectively.
A total of 47.0% of patients with PsA had FC≥80 µg/g vs. 53% of axSpA (80% axSpA-r vs. 20% axSpA-nr). The mean FC values were higher in the case of axSpA-r (395.06 µg/g) compared to the other groups (305.52 µg/g for the axSpA-nr and 306.19 µg/g for the PsA subgroup).
Overall, 10.0% of the patients had a family history of IBD and 14.6% had clinical manifestations compatible with IBD, the most common clinical manifestations were asthenia (50.0%), abdominal pain (15.6%) and chronic diarrhea (14.1%). A total of 189 colonoscopies were performed (167 in patients with FC≥80 µg/g, of which 39.7% presented some pathological finding, mostly: aphthous ulcers (65.9%), superficial ulcers (20.5%) and mucosal erythema (13.6%), mainly located in terminal ileum (41.3%).
Finally, 23 patients were diagnosed with IBD (4.4%): 22 diagnosed with Crohn's disease (95%), 1 unclassifiable IBD (5%). Of the 23 patients diagnosed with IBD, 17.4% had a family history of IBD, 30.4% had clinical symptoms compatible with IBD and 82.6% had a colonoscopy with some type of pathological finding.
Conclusion: The finding of elevated fecal calprotectin levels in patients with SpA, followed by an appropriate complementary study (endoscopic and/or radiological), allows the detection of a relevant subgroup of patients who meet diagnostic criteria for IBD.
J. Sanz: AbbVie/Abbott, 1, 6, Janssen, 1, 5, 6, Novartis, 6, UCB, 1, 5, 6; Z. Plaza: None; J. Gratacos Masmitja: AbbVie/Abbott, 1, 6, Amgen, 6, AstraZeneca, 6, Bristol-Myers Squibb(BMS), 6, Eli Lilly, 1, 6, Janssen, 1, 6, Novartis, 1, 6, Pfizer, 1, 6, UCB, 1, 6; I. Rodríguez -Lago: None; E. Trujillo: None; I. Marin-Jimenez: None; E. Perez-Pampin: None; M. Barreiro de Acosta: None; A. Aznar Esquivel: None; M. Carrillo Palau: None; M. Garcia Vivar: None; M. Muñoz: None; L. Ladehesa Pineda: None; E. Iglesias Flores: None; c. Merino: None; Y. gonzalez-Lama: AbbVie/Abbott, 1, 6, Janssen, 1, 6, Pfizer, 1, 6; M. Arévalo Salaet: None; X. calvet: None; A. Brandy-Garcia: None; M. Izquierdo Romero: None; S. MANRIQUE: None; R. Olmedo: None; J. Garcia Llorente: AbbVie/Abbott, 6, Amgen, 5, Bristol-Myers Squibb(BMS), 6, Eli Lilly, 6, Faes, 11, galápagos, 6, Gebro pharma, 5, UCB, 5; S. Pérez: None; I. Ros: Janssen, 6, novartis, 6, Pfizer, 6; N. Rull: None; J. Pinto Tasende: None; P. Ucha Abal: None; C. González: None; F. Rodríguez Martínez: None; S. Serrano Ladron de Guevara: None; M. Domínguez: None; F. Prado: None; E. González-Dávila: None; A. Gutierrez-Casbas: None.
Background/Purpose: EISER is a cross-sectional, multicenter, observational, SER-GETECCU cooperative study involving 13 Spanish hospitals whose main objective was to estimate the prevalence of undiagnosed Inflammatory Bowel Disease (IBD) in patients with spondyloarthritis (SpA), including axial (axSpA) (radiographic axSpA-r and non-radiographic; axSpA-nr) and psoriatic arthritis (PsA).
Methods: We selected patients ≥18 years of age from the NHS Rheumatology Department, SpA diagnosed according to CASPAR criteria for PsA and ASAS for axSpA. Patients under treatment with biologics were excluded. Patients currently treated with systemic steroids or in the previous 30 days were not included. Patients over 50 years of age were included if a colonoscopy wasn't performed in the last three years or, if a colonoscopy was performed but did not meet the criteria of valid colonoscopy.
Patients were recruited by the rheumatologist who collected demographic, clinical and treatment data. A fecal calprotectin (FC) determination was performed using the Quantum Blue rapid test. FC was assessed and all patients with a FC ≥80 µg/g underwent an endoscopic study by the gastroenterologist. Patients in whom the result of the endoscopic study was normal underwent to an endoscopic capsule study or magnetic resonance imaging.
Results: A total of 559 patients were included, 51.0% of whom were men. The mean age of the participants was 52.2 years, with a mean age at diagnosis of 42 years. Mean time of disease evolution was 12.3 years (Table 1).
Regarding the type of SpA, the most frequent form was axSpA-r (37.0%), followed by peripheral PsA (35.8%; Table 2). Concerning disease activity data in axSpA BASDAI 3.6, ASDAS_PCR 2.3, ASDAS_VSG 2.4 and of patients with PsA DAPSA of 10.4 (Table 2). Global assessment and mean health status reported by patients was 4.1 (0 to 10 scale) and 67.5 (0 to 100 scale; Table 2), respectively.
A total of 47.0% of patients with PsA had FC≥80 µg/g vs. 53% of axSpA (80% axSpA-r vs. 20% axSpA-nr). The mean FC values were higher in the case of axSpA-r (395.06 µg/g) compared to the other groups (305.52 µg/g for the axSpA-nr and 306.19 µg/g for the PsA subgroup).
Overall, 10.0% of the patients had a family history of IBD and 14.6% had clinical manifestations compatible with IBD, the most common clinical manifestations were asthenia (50.0%), abdominal pain (15.6%) and chronic diarrhea (14.1%). A total of 189 colonoscopies were performed (167 in patients with FC≥80 µg/g, of which 39.7% presented some pathological finding, mostly: aphthous ulcers (65.9%), superficial ulcers (20.5%) and mucosal erythema (13.6%), mainly located in terminal ileum (41.3%).
Finally, 23 patients were diagnosed with IBD (4.4%): 22 diagnosed with Crohn's disease (95%), 1 unclassifiable IBD (5%). Of the 23 patients diagnosed with IBD, 17.4% had a family history of IBD, 30.4% had clinical symptoms compatible with IBD and 82.6% had a colonoscopy with some type of pathological finding.
Conclusion: The finding of elevated fecal calprotectin levels in patients with SpA, followed by an appropriate complementary study (endoscopic and/or radiological), allows the detection of a relevant subgroup of patients who meet diagnostic criteria for IBD.

Table 1: Sociodemographic and general data of all patients included in the study.

Table 2: Type and activity of patients enrolled in the study.
J. Sanz: AbbVie/Abbott, 1, 6, Janssen, 1, 5, 6, Novartis, 6, UCB, 1, 5, 6; Z. Plaza: None; J. Gratacos Masmitja: AbbVie/Abbott, 1, 6, Amgen, 6, AstraZeneca, 6, Bristol-Myers Squibb(BMS), 6, Eli Lilly, 1, 6, Janssen, 1, 6, Novartis, 1, 6, Pfizer, 1, 6, UCB, 1, 6; I. Rodríguez -Lago: None; E. Trujillo: None; I. Marin-Jimenez: None; E. Perez-Pampin: None; M. Barreiro de Acosta: None; A. Aznar Esquivel: None; M. Carrillo Palau: None; M. Garcia Vivar: None; M. Muñoz: None; L. Ladehesa Pineda: None; E. Iglesias Flores: None; c. Merino: None; Y. gonzalez-Lama: AbbVie/Abbott, 1, 6, Janssen, 1, 6, Pfizer, 1, 6; M. Arévalo Salaet: None; X. calvet: None; A. Brandy-Garcia: None; M. Izquierdo Romero: None; S. MANRIQUE: None; R. Olmedo: None; J. Garcia Llorente: AbbVie/Abbott, 6, Amgen, 5, Bristol-Myers Squibb(BMS), 6, Eli Lilly, 6, Faes, 11, galápagos, 6, Gebro pharma, 5, UCB, 5; S. Pérez: None; I. Ros: Janssen, 6, novartis, 6, Pfizer, 6; N. Rull: None; J. Pinto Tasende: None; P. Ucha Abal: None; C. González: None; F. Rodríguez Martínez: None; S. Serrano Ladron de Guevara: None; M. Domínguez: None; F. Prado: None; E. González-Dávila: None; A. Gutierrez-Casbas: None.



