Poster Session A
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (0510–0542) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I: AxSpA
0521: Work Productivity Improved in Patients with Axial Spondyloarthritis Receiving Bimekizumab Treatment over 52 Weeks: Results from Two Phase 3 Studies
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- MR
Martin Rudwaleit, MD
Klinikum Bielefeld Rosenhoehe, Bielefeld University
Bielefeld, GermanyDisclosure information not submitted.
Abstract Poster Presenter(s)
Martin Rudwaleit1, Victoria Navarro-Compán2, Atul Deodhar3, Maureen Dubreuil4, Michael Frank Mørup5, Vanessa Taieb6, Christine de la Loge7, Carmen Fleurinck8, Ute Massow9, Thomas Vaux10 and Annelies Boonen11, 1University of Bielefeld, Klinikum Bielefeld, Bielefeld, Germany, 2Department of Rheumatology, La Paz University Hospital, IdiPaz, Madrid, Spain, 3Division of Arthritis and Rheumatic Disease, Oregon Health & Science University, Portland, OR, 4Department of Rheumatology, Boston University School of Medicine, Milton, MA, 5UCB Pharma, Copenhagen, Denmark, 6UCB Pharma, Colombes, France, 7UCB Pharma, Brussels, Belgium, 8UCB Pharma, Oosterzele, Belgium, 9UCB Pharma, Monheim am Rhein, Germany, 10UCB Pharma, Slough, United Kingdom, 11Care and Public Health Research Institute (Caphri), Maastricht University, Maastricht, Netherlands
Background/Purpose: The clinical manifestations of axial spondyloarthritis (axSpA) limit physical function and work productivity, posing an economic burden to patients (pts) and society.1 Bimekizumab (BKZ), a monoclonal IgG1 antibody that selectively inhibits IL-17F in addition to IL-17A, demonstrated sustained efficacy in controlling disease activity up to Week (Wk) 52 in pts with non-radiographic (nr-) and radiographic (r-)axSpA (i.e., AS)2 in the phase 3 studies BE MOBILE 1 and 2, respectively.3 This analysis assessed the impact of BKZ on work productivity and activity impairment (WPAI) at Wk 16 and Wk 52 in pts across the full disease spectrum of axSpA.
Methods: The parallel BE MOBILE 1 (NCT03928704) and 2 (NCT03928743) studies comprised a 16-wk double-blind period followed by a 36-wk maintenance period.3 Pts were randomized to subcutaneous BKZ 160 mg every 4 wks (Q4W) or placebo (PBO). All pts received BKZ 160 mg Q4W from Wk 16. We report mean change from baseline (BL) at Wk 16 and 52 in WPAI Specific Health Problem scores,4 which assess disease impact on pts' impairment while at paid work (i.e., presenteeism), work time missed (i.e., absenteeism, including sick leave), overall work impairment (composite of presenteeism and absenteeism) and daily activity impairment (outside paid work). Observed case data are reported.
Results: Almost 75% of pts were employed at BL (nr-axSpA BKZ: 95/128 [74.2%], PBO: 93/126 [73.8%]; r-axSpA BKZ: 161/221 [72.9%], PBO: 82/111 [73.9%]). Pts had substantial overall work impairment at BL, with presenteeism contributing most to this (Table).
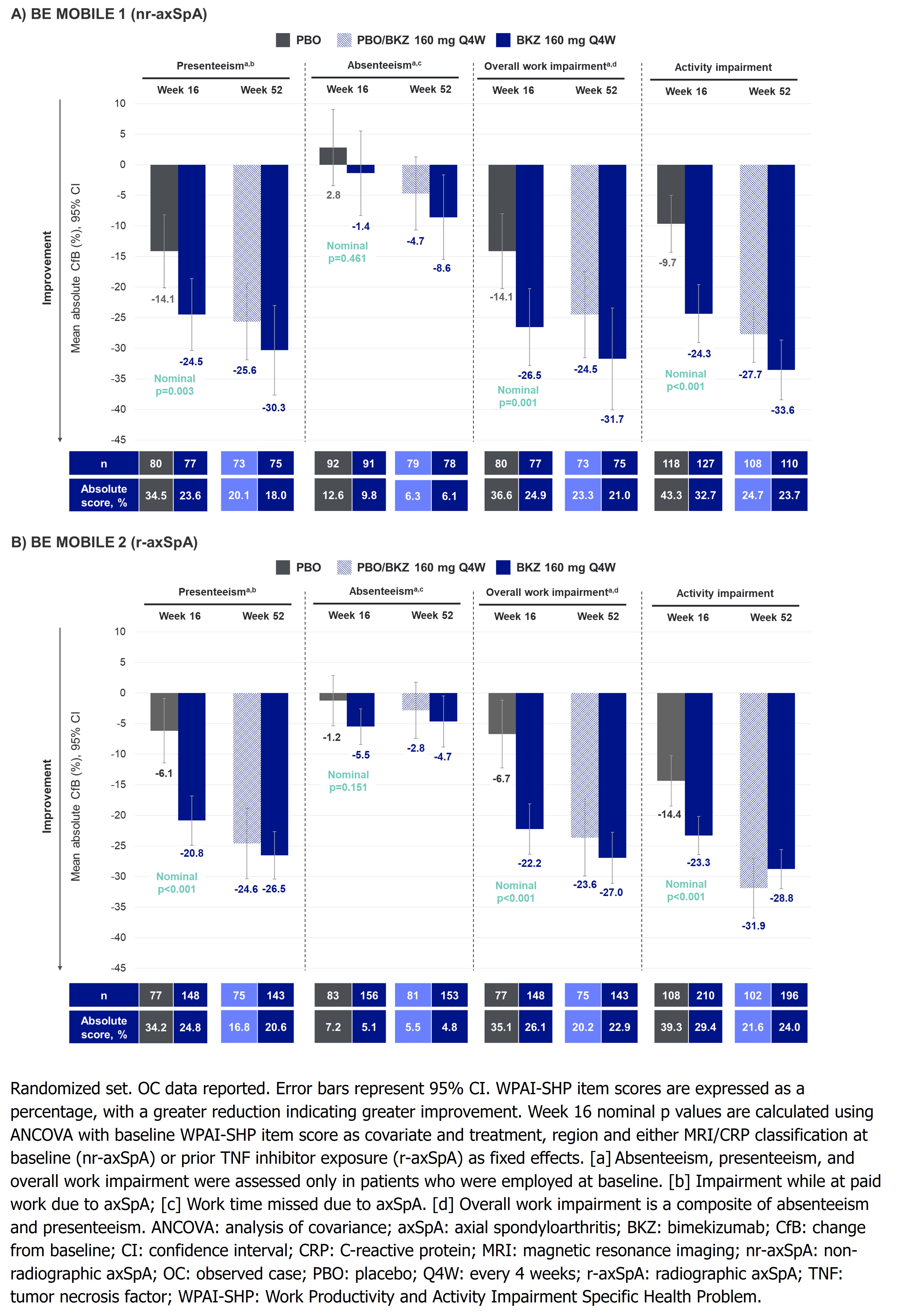
At Wk 16, mean reductions from BL (i.e., absolute improvement) were greater in BKZ- vs PBO-randomized pts for presenteeism (nr-axSpA: –24.5% vs –14.1%, nominal p=0.003; r-axSpA: –20.8% vs –6.1%, nominal p< 0.001), overall work impairment (nr-axSpA: –26.5% vs –14.1%, nominal p=0.001; r-axSpA: –22.2% vs –6.7%, nominal p< 0.001) and activity impairment (nr-axSpA: –24.3% vs –9.7%; r-axSpA: –23.3% vs –14.4%; nominal p< 0.001; Figure). Mean improvements in overall work impairment were sustained or further improved to Wk 52 in BKZ-randomized pts (nr-axSpA: –31.7%; r-axSpA: –27.0%); pts who switched from PBO to BKZ at Wk 16 reached similar levels of improvement to BKZ-randomized pts at Wk 52. Similar trends were seen in presenteeism and activity impairment (Figure).
Mean BL absenteeism scores were low compared with other WPAI items (Table), leaving limited room for improvement. At Wk 16, no clear separation was seen for improvements in absenteeism between BKZ-randomized pts and PBO; this response improved or was sustained in BKZ-randomized pts at Wk 52 (Figure). Absenteeism may also have been impacted by the COVID-19 pandemic.
Conclusion: BKZ treatment resulted in substantial improvements in overall work and activity impairment at Wk 16, with further improvements at Wk 52 in pts across the full disease spectrum of axSpA.
References:1. Strand V. J Clin Rheumatol 2017;23:383–91; 2. Baraliakos X. Arthritis Rheumatol 2022;74(suppl 9); 3. Boel A. Ann Rheum Dis 2019;78:1545–9; 4. Hoepken B. Qual Life Res. 2021;30:945–54.
.jpg)
M. Rudwaleit: AbbVie, 2, 6, Boehringer Ingelheim, 6, Chugai, 6, Eli Lilly, 2, 6, Janssen, 6, Novartis, 2, 6, Pfizer, 6, UCB Pharma, 2, 6; V. Navarro-Compán: AbbVie, 2, 5, 6, Eli Lilly, 2, 6, Galapagos, 2, Janssen, 6, MoonLake, 2, MSD, 2, 6, Novartis, 2, 5, 6, Pfizer, 2, 6, UCB, 2, 6; A. Deodhar: AbbVie, 2, 5, Amgen, 2, Aurinia, 2, Bristol Myers Squibb, 2, 5, Celgene, 5, Eli Lilly, 2, 5, Janssen, 2, 6, MoonLake, 2, 5, Novartis, 2, 5, 6, Pfizer Inc, 2, 5, 6, UCB, 2, 5; M. Dubreuil: Amgen, 2, Pfizer, 5, UCB Pharma, 2; M. Mørup: UCB Pharma, 3; V. Taieb: UCB Pharma, 3, 11; C. de la Loge: UCB Pharma, 2; C. Fleurinck: UCB Pharma, 3; U. Massow: UCB Pharma, 3; T. Vaux: UCB Pharma, 3; A. Boonen: AbbVie, 2, 5, 6, Galapagos, 2, 6, Novartis, 2, 6, Pfizer, 5, 6, UCB Pharma, 2, 6.
Background/Purpose: The clinical manifestations of axial spondyloarthritis (axSpA) limit physical function and work productivity, posing an economic burden to patients (pts) and society.1 Bimekizumab (BKZ), a monoclonal IgG1 antibody that selectively inhibits IL-17F in addition to IL-17A, demonstrated sustained efficacy in controlling disease activity up to Week (Wk) 52 in pts with non-radiographic (nr-) and radiographic (r-)axSpA (i.e., AS)2 in the phase 3 studies BE MOBILE 1 and 2, respectively.3 This analysis assessed the impact of BKZ on work productivity and activity impairment (WPAI) at Wk 16 and Wk 52 in pts across the full disease spectrum of axSpA.
Methods: The parallel BE MOBILE 1 (NCT03928704) and 2 (NCT03928743) studies comprised a 16-wk double-blind period followed by a 36-wk maintenance period.3 Pts were randomized to subcutaneous BKZ 160 mg every 4 wks (Q4W) or placebo (PBO). All pts received BKZ 160 mg Q4W from Wk 16. We report mean change from baseline (BL) at Wk 16 and 52 in WPAI Specific Health Problem scores,4 which assess disease impact on pts' impairment while at paid work (i.e., presenteeism), work time missed (i.e., absenteeism, including sick leave), overall work impairment (composite of presenteeism and absenteeism) and daily activity impairment (outside paid work). Observed case data are reported.
Results: Almost 75% of pts were employed at BL (nr-axSpA BKZ: 95/128 [74.2%], PBO: 93/126 [73.8%]; r-axSpA BKZ: 161/221 [72.9%], PBO: 82/111 [73.9%]). Pts had substantial overall work impairment at BL, with presenteeism contributing most to this (Table).
At Wk 16, mean reductions from BL (i.e., absolute improvement) were greater in BKZ- vs PBO-randomized pts for presenteeism (nr-axSpA: –24.5% vs –14.1%, nominal p=0.003; r-axSpA: –20.8% vs –6.1%, nominal p< 0.001), overall work impairment (nr-axSpA: –26.5% vs –14.1%, nominal p=0.001; r-axSpA: –22.2% vs –6.7%, nominal p< 0.001) and activity impairment (nr-axSpA: –24.3% vs –9.7%; r-axSpA: –23.3% vs –14.4%; nominal p< 0.001; Figure). Mean improvements in overall work impairment were sustained or further improved to Wk 52 in BKZ-randomized pts (nr-axSpA: –31.7%; r-axSpA: –27.0%); pts who switched from PBO to BKZ at Wk 16 reached similar levels of improvement to BKZ-randomized pts at Wk 52. Similar trends were seen in presenteeism and activity impairment (Figure).
Mean BL absenteeism scores were low compared with other WPAI items (Table), leaving limited room for improvement. At Wk 16, no clear separation was seen for improvements in absenteeism between BKZ-randomized pts and PBO; this response improved or was sustained in BKZ-randomized pts at Wk 52 (Figure). Absenteeism may also have been impacted by the COVID-19 pandemic.
Conclusion: BKZ treatment resulted in substantial improvements in overall work and activity impairment at Wk 16, with further improvements at Wk 52 in pts across the full disease spectrum of axSpA.
References:1. Strand V. J Clin Rheumatol 2017;23:383–91; 2. Baraliakos X. Arthritis Rheumatol 2022;74(suppl 9); 3. Boel A. Ann Rheum Dis 2019;78:1545–9; 4. Hoepken B. Qual Life Res. 2021;30:945–54.
.jpg)
Table. Baseline employment and WPAI-SHP item scores (OC)

Figure. Mean absolute change from baseline in WPAI-SHP items at Week 16 and Week 52 (OC)
M. Rudwaleit: AbbVie, 2, 6, Boehringer Ingelheim, 6, Chugai, 6, Eli Lilly, 2, 6, Janssen, 6, Novartis, 2, 6, Pfizer, 6, UCB Pharma, 2, 6; V. Navarro-Compán: AbbVie, 2, 5, 6, Eli Lilly, 2, 6, Galapagos, 2, Janssen, 6, MoonLake, 2, MSD, 2, 6, Novartis, 2, 5, 6, Pfizer, 2, 6, UCB, 2, 6; A. Deodhar: AbbVie, 2, 5, Amgen, 2, Aurinia, 2, Bristol Myers Squibb, 2, 5, Celgene, 5, Eli Lilly, 2, 5, Janssen, 2, 6, MoonLake, 2, 5, Novartis, 2, 5, 6, Pfizer Inc, 2, 5, 6, UCB, 2, 5; M. Dubreuil: Amgen, 2, Pfizer, 5, UCB Pharma, 2; M. Mørup: UCB Pharma, 3; V. Taieb: UCB Pharma, 3, 11; C. de la Loge: UCB Pharma, 2; C. Fleurinck: UCB Pharma, 3; U. Massow: UCB Pharma, 3; T. Vaux: UCB Pharma, 3; A. Boonen: AbbVie, 2, 5, 6, Galapagos, 2, 6, Novartis, 2, 6, Pfizer, 5, 6, UCB Pharma, 2, 6.



