Poster Session B
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (1412–1441) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II: SpA
1419: Impact of Psoriatic Arthritis Manifestations on Perception of Pain Improvement: Pooled Analysis of Two Phase 3, Randomized, Double-Blind, Placebo-Controlled Studies with Guselkumab
Monday, November 13, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall

Peter Nash, FRACP
Griffith UNiversity
Sunshine Coast, Queensland, AustraliaDisclosure information not submitted.
Abstract Poster Presenter(s)
Peter Nash1, Iain McInnes2, Christopher T. Ritchlin3, Lai-Shan Tam4, Enrique Soriano5, Michael Starr6, Emmanouil Rampakakis7, Frederic Lavie8, May Shawi9, Xenofon Baraliakos10 and Philip J. Mease11, 1School of Medicine, Griffith University, Brisbane, Australia, 2University of Glasgow, Glasgow, United Kingdom, 3University of Rochester Medical Center, Department of Medicine – Allergy/Immunology and Rheumatology, Rochester, NY, 4The Chinese University of Hong Kong, New Territories, China, 5Rheumatology Section, Internal Medicine Services, Hospital Italiano de Buenos Aires, and University Institute Hospital Italiano de Buenos Aires, Buenos Aires, Argentina, 6Division of Rheumatology, McGill University Health Center, Montreal, QC, Canada, 7McGill University, Department of Pediatrics / JSS Medical Research, Scientific Affairs, Montreal, QC, Canada, 8The Janssen Pharmaceutical Companies of Johnson & Johnson, Paris, France, 9Immunology, Janssen Research & Development, LLC, Titusville, NJ, 10Rheumazentrum Ruhrgebiet Herne, Ruhr-University Bochum, Herne, Germany, 11Swedish Medical Center/Providence St. Joseph Health and University of Washington School of Medicine, Seattle, WA
Background/Purpose: Pain in PsA has multifaceted origins (e.g., peripheral joint inflammation, axial involvement [axPsA], skin lesions, dactylitis, enthesitis, underlying conditions) and can be difficult to treat. Guselkumab (GUS), a fully human IL-23p19 subunit inhibitor, is effective in treating multiple PsA domains and elicited durable improvement in patient-reported pain (PtP) in the DISCOVER-1&2 trials1,2. Here, we assessed association between improvement in key PsA manifestations and PtP using 1-year DISCOVER-1&2 data.
Methods: DISCOVER-1&2 enrolled adults with active PsA despite standard therapies3,4. Patients were randomized 1:1:1 to GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at W0, W4, then Q8W; or placebo with crossover to GUS 100 mg Q4W at W24. Treatment groups were pooled (N=1120). Longitudinal associations of improvement in swollen joint count (SJC,0-66), tender joint count (TJC, 0-68), Leeds enthesitis index (LEI), dactylitis severity score, Psoriasis Area and Severity Index (PASI), axPsA (N=312), and improvement in overall PtP (0-100 mm) and spinal pain (BASDAI question 2 in patients with axPsA) were assessed. Longitudinal associations of improvement in these PsA manifestations with ≥30%/50%/70% improvements in PtP (PtP-30/50/70) were assessed.
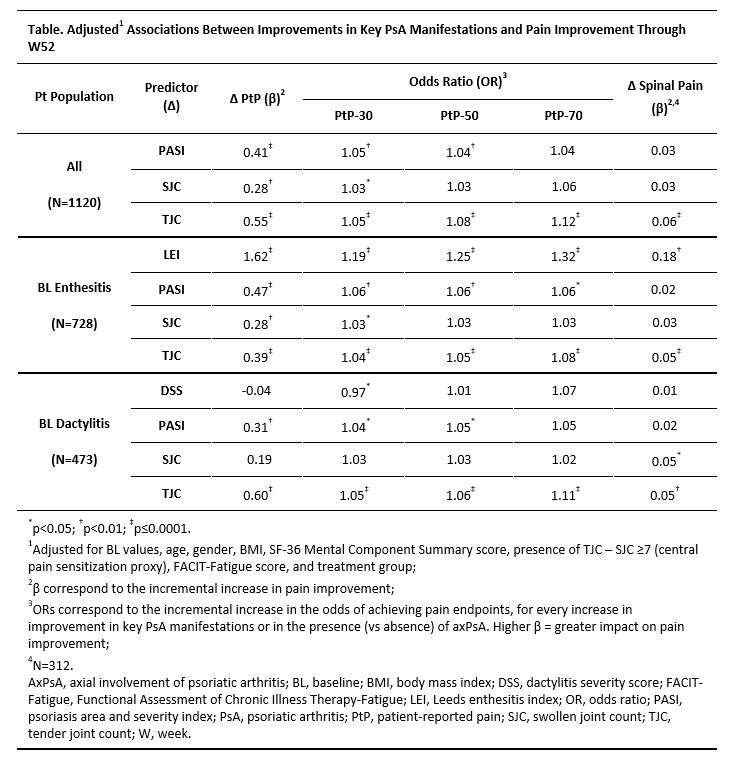
Results: Mean (SD) baseline PtP of 61.2 (19.8) indicated substantial burden. Upon adjusting for potential confounders, greater improvement in PASI, SJC, and TJC (mutually adjusted) were each associated with significantly greater improvement in PtP and higher odds of achieving PtP-30 through W52 (Table). PASI reduction was also associated with greater odds of PtP-50, as was TJC improvement for PtP-50/70. In patients with baseline enthesitis, improvements in LEI, PASI, and TJC were each associated with greater PtP improvement and attaining PtP-30/50/70; SJC reduction was only associated with PtP-30. In patients with baseline dactylitis, PASI and TJC reductions were significantly associated with PtP improvement. Overall, axPsA presence did not impact the extent of PtP improvement (data not shown). In patients with axPsA, significant associations were observed between spinal pain improvement and TJC and LEI improvement.
Conclusion: Improvements in key PsA manifestations were significantly associated with pain reduction, although to varying extents. TJC reduction had the greatest impact on PtP improvement, likely due to overlap of the construct measured. Psoriasis improvement had a greater impact on pain relief than SJC improvement, highlighting the sensory burden of skin lesions, while enthesitis improvement showed a significant association with both overall and spinal pain relief. These findings underscore the importance of utilizing treatments effective across manifestations to address recalcitrant PsA symptoms.
References:
1. Ritchlin CT. RMD Open 2022;8:e002195
2. Nash P. ACR Convergence 2021 (PO1333)
3. Deodhar A. Lancet 2020;395:1115
4. Mease PJ. Lancet 2020;395:1126
P. Nash: AbbVie, 5, 6, Bristol Myers Squibb, 5, 6, Celgene, 5, 6, Eli Lilly, 5, 6, Galapagos, 5, 6, GSK, 5, 6, Janssen, 5, 6, Novartis, 5, 6, Pfizer Inc, 5, 6; I. McInnes: AbbVie, 2, Amgen, 2, AstraZeneca, 2, Bristol Myers Squibb, 2, 5, Cabaletta, 2, 11, Causeway Therapeutics, 2, 11, Celgene, 2, 5, Compugen, 2, 11, Dextera, 11, Eli Lilly, 2, EveloBio, 1, 2, 4, 11, Gilead, 2, Janssen, 2, 5, Moonlake, 2, NHS GGC, 4, Novartis, 2, 5, Pfizer, 2, Sanofi, 2, UCB, 2, 5, Versus Arthritis, 12, Trustee Status; C. T. Ritchlin: AbbVie, 2, 5, Amgen, 2, 5, Eli Lilly, 2, Gilead, 2, Janssen, 2, Novartis, 2, Pfizer, 2, UCB, 2, 5; L. Tam: AbbVie, 5, Amgen, 2, 5, Boehringer-Ingelheim, 2, 5, Eli Lilly, 2, GSK, 5, Janssen, 2, 5, Novartis, 5, Pfizer, 2, 5, Sanofi, 2; E. Soriano: AbbVie, 2, 5, 6, Amgen, 6, Bristol-Myers Squibb, 6, Eli Lilly, 6, Janssen, 2, 5, 6, Novartis, 2, 5, 6, Pfizer, 5, 6, Roche, 2, 5, 6, UCB, 5, 6; M. Starr: AbbVie, 1, 6, Eli Lilly, 1, 6, Janssen, 1, 6, Novartis, 1, 6, Pfizer, 1, 6, UCB, 1, 6; E. Rampakakis: Janssen, 2, JSS Medical Research, Inc, 3; F. Lavie: Janssen, 3, Johnson & Johnson, 11; M. Shawi: Immunology Global Medical Affairs, Janssen Pharmaceutical Companies of Johnson & Johnson, 3, Johnson & Johnson, 11; X. Baraliakos: AbbVie, 2, 6, BMS, 2, 6, Chugai, 2, 6, Eli Lilly, 2, 6, Galapagos, 2, 6, Gilead, 2, 6, MSD, 2, 6, Novartis, 2, 6, Pfizer Inc, 2, 6, UCB, 2, 6; P. Mease: AbbVie, 2, 5, 6, Acelyrin, 2, Aclaris, 2, Amgen, 2, 5, 6, Boehringer Ingelheim, 2, Bristol Myers Squibb, 2, 5, Eli Lilly, 2, 5, 6, Galapagos, 2, Gilead, 2, GlaxoSmithKline, 2, Inmagene, 2, Janssen, 2, 5, 6, MoonLake Pharma, 2, Novartis, 2, 5, 6, Pfizer, 2, 5, 6, Sun Pharma, 2, 5, UCB Pharma, 2, 5, 6, Ventyx, 2, Xinthera, 2.
Background/Purpose: Pain in PsA has multifaceted origins (e.g., peripheral joint inflammation, axial involvement [axPsA], skin lesions, dactylitis, enthesitis, underlying conditions) and can be difficult to treat. Guselkumab (GUS), a fully human IL-23p19 subunit inhibitor, is effective in treating multiple PsA domains and elicited durable improvement in patient-reported pain (PtP) in the DISCOVER-1&2 trials1,2. Here, we assessed association between improvement in key PsA manifestations and PtP using 1-year DISCOVER-1&2 data.
Methods: DISCOVER-1&2 enrolled adults with active PsA despite standard therapies3,4. Patients were randomized 1:1:1 to GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at W0, W4, then Q8W; or placebo with crossover to GUS 100 mg Q4W at W24. Treatment groups were pooled (N=1120). Longitudinal associations of improvement in swollen joint count (SJC,0-66), tender joint count (TJC, 0-68), Leeds enthesitis index (LEI), dactylitis severity score, Psoriasis Area and Severity Index (PASI), axPsA (N=312), and improvement in overall PtP (0-100 mm) and spinal pain (BASDAI question 2 in patients with axPsA) were assessed. Longitudinal associations of improvement in these PsA manifestations with ≥30%/50%/70% improvements in PtP (PtP-30/50/70) were assessed.
Results: Mean (SD) baseline PtP of 61.2 (19.8) indicated substantial burden. Upon adjusting for potential confounders, greater improvement in PASI, SJC, and TJC (mutually adjusted) were each associated with significantly greater improvement in PtP and higher odds of achieving PtP-30 through W52 (Table). PASI reduction was also associated with greater odds of PtP-50, as was TJC improvement for PtP-50/70. In patients with baseline enthesitis, improvements in LEI, PASI, and TJC were each associated with greater PtP improvement and attaining PtP-30/50/70; SJC reduction was only associated with PtP-30. In patients with baseline dactylitis, PASI and TJC reductions were significantly associated with PtP improvement. Overall, axPsA presence did not impact the extent of PtP improvement (data not shown). In patients with axPsA, significant associations were observed between spinal pain improvement and TJC and LEI improvement.
Conclusion: Improvements in key PsA manifestations were significantly associated with pain reduction, although to varying extents. TJC reduction had the greatest impact on PtP improvement, likely due to overlap of the construct measured. Psoriasis improvement had a greater impact on pain relief than SJC improvement, highlighting the sensory burden of skin lesions, while enthesitis improvement showed a significant association with both overall and spinal pain relief. These findings underscore the importance of utilizing treatments effective across manifestations to address recalcitrant PsA symptoms.
References:
1. Ritchlin CT. RMD Open 2022;8:e002195
2. Nash P. ACR Convergence 2021 (PO1333)
3. Deodhar A. Lancet 2020;395:1115
4. Mease PJ. Lancet 2020;395:1126

P. Nash: AbbVie, 5, 6, Bristol Myers Squibb, 5, 6, Celgene, 5, 6, Eli Lilly, 5, 6, Galapagos, 5, 6, GSK, 5, 6, Janssen, 5, 6, Novartis, 5, 6, Pfizer Inc, 5, 6; I. McInnes: AbbVie, 2, Amgen, 2, AstraZeneca, 2, Bristol Myers Squibb, 2, 5, Cabaletta, 2, 11, Causeway Therapeutics, 2, 11, Celgene, 2, 5, Compugen, 2, 11, Dextera, 11, Eli Lilly, 2, EveloBio, 1, 2, 4, 11, Gilead, 2, Janssen, 2, 5, Moonlake, 2, NHS GGC, 4, Novartis, 2, 5, Pfizer, 2, Sanofi, 2, UCB, 2, 5, Versus Arthritis, 12, Trustee Status; C. T. Ritchlin: AbbVie, 2, 5, Amgen, 2, 5, Eli Lilly, 2, Gilead, 2, Janssen, 2, Novartis, 2, Pfizer, 2, UCB, 2, 5; L. Tam: AbbVie, 5, Amgen, 2, 5, Boehringer-Ingelheim, 2, 5, Eli Lilly, 2, GSK, 5, Janssen, 2, 5, Novartis, 5, Pfizer, 2, 5, Sanofi, 2; E. Soriano: AbbVie, 2, 5, 6, Amgen, 6, Bristol-Myers Squibb, 6, Eli Lilly, 6, Janssen, 2, 5, 6, Novartis, 2, 5, 6, Pfizer, 5, 6, Roche, 2, 5, 6, UCB, 5, 6; M. Starr: AbbVie, 1, 6, Eli Lilly, 1, 6, Janssen, 1, 6, Novartis, 1, 6, Pfizer, 1, 6, UCB, 1, 6; E. Rampakakis: Janssen, 2, JSS Medical Research, Inc, 3; F. Lavie: Janssen, 3, Johnson & Johnson, 11; M. Shawi: Immunology Global Medical Affairs, Janssen Pharmaceutical Companies of Johnson & Johnson, 3, Johnson & Johnson, 11; X. Baraliakos: AbbVie, 2, 6, BMS, 2, 6, Chugai, 2, 6, Eli Lilly, 2, 6, Galapagos, 2, 6, Gilead, 2, 6, MSD, 2, 6, Novartis, 2, 6, Pfizer Inc, 2, 6, UCB, 2, 6; P. Mease: AbbVie, 2, 5, 6, Acelyrin, 2, Aclaris, 2, Amgen, 2, 5, 6, Boehringer Ingelheim, 2, Bristol Myers Squibb, 2, 5, Eli Lilly, 2, 5, 6, Galapagos, 2, Gilead, 2, GlaxoSmithKline, 2, Inmagene, 2, Janssen, 2, 5, 6, MoonLake Pharma, 2, Novartis, 2, 5, 6, Pfizer, 2, 5, 6, Sun Pharma, 2, 5, UCB Pharma, 2, 5, 6, Ventyx, 2, Xinthera, 2.



