Poster Session A
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (0510–0542) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I: AxSpA
0520: How Do Early Disease Activity and Early Clinical Response Associate with Long-Term Outcomes with Ixekizumab in Radiographic Axial Spondyloarthritis?
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- SR
Sofia Ramiro, MD, PhD
Leiden University Medical Center
Bunde, NetherlandsDisclosure information not submitted.
Abstract Poster Presenter(s)
Sofia Ramiro1, Cedric Lukas2, Louis Bessette3, Pendleton Wickersham4, Soyi Liu-Leage5, Tommaso Panni5, Rebecca Bolce5, Boris Janos5, Michael Nissen6 and James Cheng-Chung Wei7, 1Department of Rheumatology, Leiden University Medical Center, Leiden, Netherlands, 2CHU Montpellier, Montpellier, France, 3Centre de l'Ostéoporose et de Rhumatologie de Québec, Quebec City, QC, Canada, 4Arthritis Associates PA, San Antonio, TX, 5Eli Lilly and Company, Indianapolis, IN, 6Geneva University Hospitals, Geneva, Switzerland, 7Chung Shan Medical University Hospital, Department of Rheumatology, Taichung, Taiwan
Background/Purpose: The ASAS-EULAR recommendations for the management of axial spondyloarthritis (axSpA) advise that a patient be assessed for biological disease-modifying anti-rheumatic drug (bDMARD) treatment response after at least 12 weeks of treatment1. The recommended treatment target for axSpA is inactive disease (ID) or low disease activity (LDA)2, as together defined by an Ankylosing Spondylitis Disease Activity Score (ASDAS) <2.13. This analysis aimed to explore the association between treatment response at Week (W)12 and W24, and attainment of the ASDAS < 2.1 treat-to-target (T2T) recommendation at W52 in patients with radiographic (r-) axSpA treated with ixekizumab (IXE).
Methods: This post hoc analysis included patients randomly assigned to IXE 80mg every 4 weeks (N=81) from COAST-V (NCT02696785), a Phase 3 trial that investigated the efficacy of IXE in bDMARD-naïve patients with r-axSpA. The proportion of patients who achieved the ASDAS < 2.1 target response at W52 was evaluated among those in ID, LDA, high disease activity (HDA) or very high disease activity (VHDA) at W12 or W24. The proportion of patients who achieved ASDAS< 2.1 at W52 was also measured among those who attained meaningful clinical response at W12 or W24, as defined by a clinically important (CII, ∆ASDAS ≥1.1) or a major improvement (MI, ∆ASDAS ≥2.0) in ASDAS, improvement of ≥50% in Bath Ankylosing Spondylitis Disease Activity Index score (BASDAI50), or achievement of BASDAI < 4. Patient and disease characteristics are described according to responses at W12 or W24 and W52. Non-responder imputation was used to handle missing data.
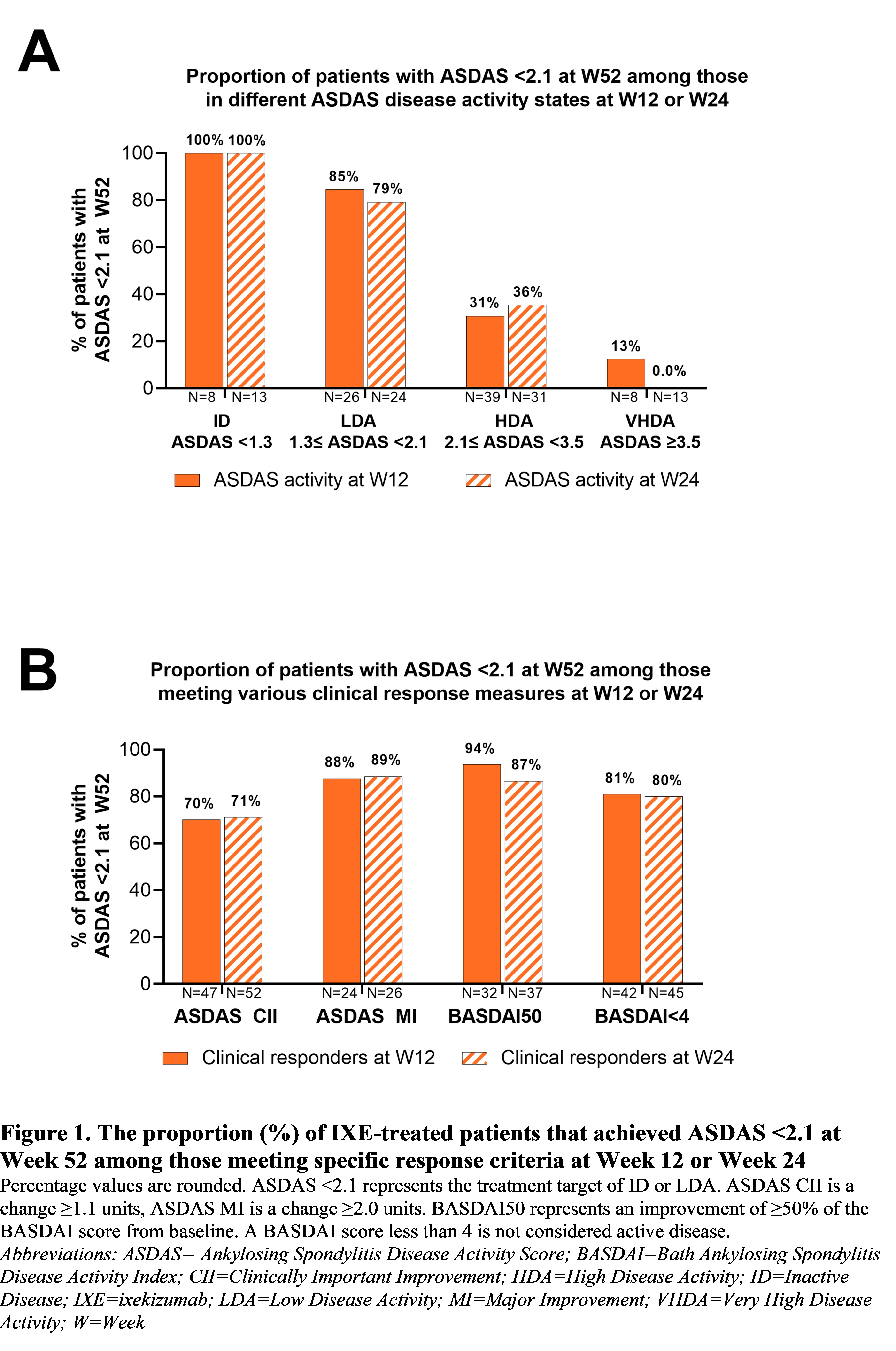
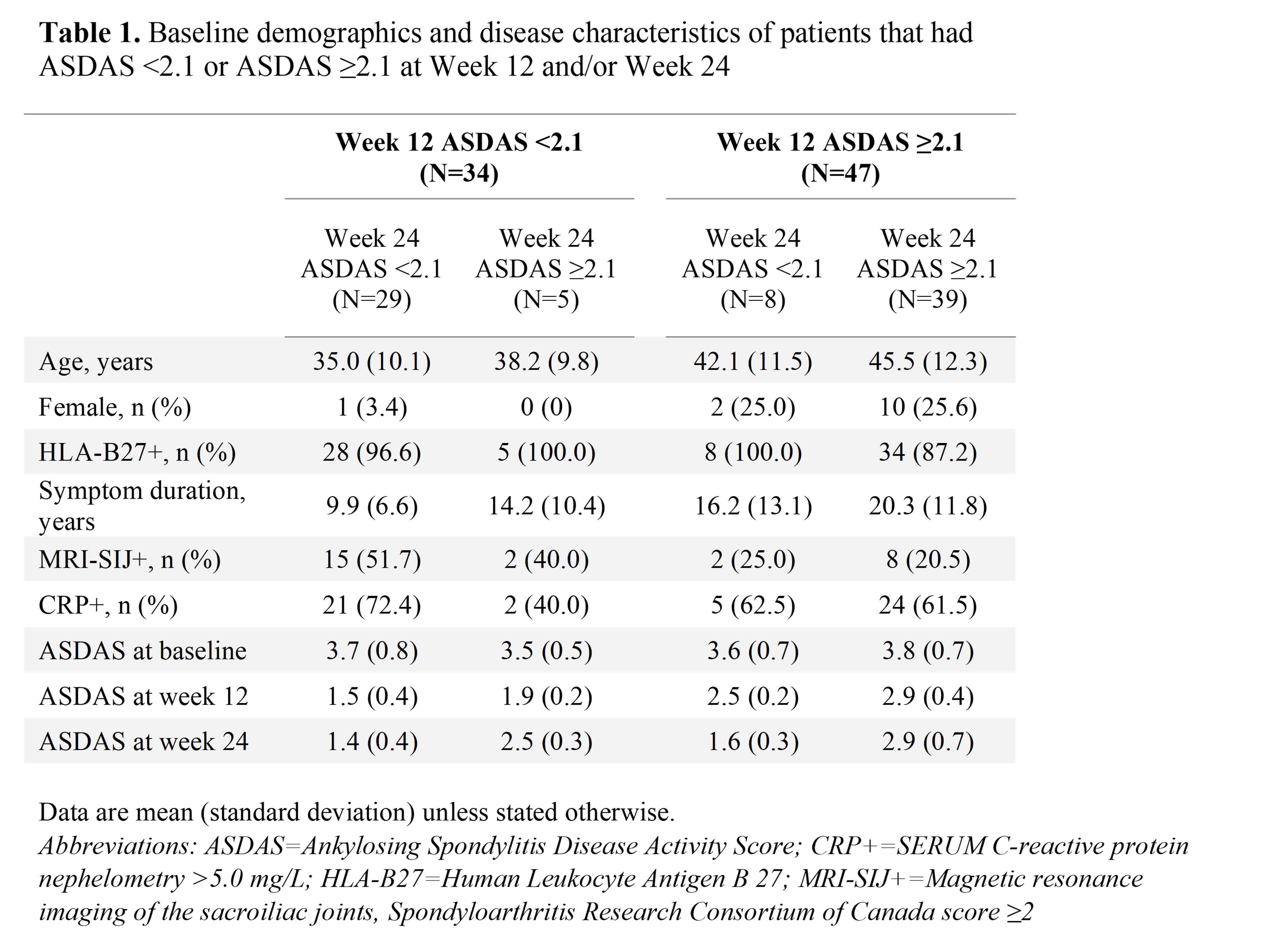
Results: Of the 81 patients, 34 (42%) were in ID or LDA at W12 (8 ID, 26 LDA) and most of these patients met the ASDAS < 2.1 target at W52 (ID=100%, LDA=85%; Figure 1A). 47 patients achieved ASDAS CII at W12 and of those, 33 (70%) were in ID or LDA at W52 (Figure 1B). Similarly, most patients who met the ASDAS MI, BASDAI50 or BASDAI< 4 clinical response measures at W12 met ASDAS < 2.1 at W52 (81–94%; Figure 1B). 37 patients (46%) were in ID or LDA at W24 (13 ID, 24 LDA) and of those, 29 (78%) had already achieved ASDAS < 2.1 at W12 (Table 1), and most also met ASDAS < 2.1 at W52 (ID=100%, LDA=79%; Figure 1A). 52 patients achieved ASDAS CII at W24 and among them, 37 (71%) were in ID or LDA at W52 (Figure 1B). Most patients who met the ASDAS MI, BASDAI50 or BASDAI< 4 clinical response measures at W24 met ASDAS < 2.1 at W52 (80–89%; Figure 1B). Patients who did not meet ASDAS < 2.1 at W12 were more likely to be older, female, or with a longer symptom duration versus those who did, and 8 of these 47 patients (17%) subsequently achieved ASDAS < 2.1 at W24 (Table 1). 39 patients were in HDA or VHDA (ASDAS ≥2.1) at both W12 and W24 (Table 1), and 9 (23%) of these patients (11% of the 81 included patients) met the ASDAS < 2.1 target at W52.
Conclusion: These data regarding IXE-treated patients with r-axSpA reinforce the current ASAS-EULAR and T2T recommendations, in that those who achieved ASDAS CII or ASDAS < 2.1 at W12 and/or W24 were highly likely to attain the treatment target of ID or LDA at W52.
References
1. Ramiro SE et al. Ann Rheum Dis. 2023; 82(1):19-34
2. Smolen JS et al. Ann Rheum Dis. 2018; 77(1): 3-17
3. Machado PM et al. Ann Rheum Dis. 2018; 77(10): 1539-1540
S. Ramiro: AbbVie, 2, 5, Eli Lilly, 2, Galapagos, 5, MSD, 2, 5, Novartis, 2, 5, Pfizer, 2, 5, Sanofi, 2, UCB Pharma, 2, 5; C. Lukas: Abbvie, 2, 6, Amgen, 2, 6, Biogen, 2, 6, BMS, 2, 6, Eli Lilly, 2, 6, Janssen, 2, 6, MSD, 2, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche Chugai, 2, 6, UCB, 2, 6; L. Bessette: AbbVie, 2, 5, 6, Amgen, 2, 5, 6, Bristol Myers Squibb, 2, 5, 6, Eli Lilly, 2, 5, 6, Fresenius Kabi, 2, 6, Gilead, 2, 5, 6, JAMP Pharma, 2, 5, 6, Janssen, 2, 5, 6, Novartis, 2, 5, 6, Organon, 2, 6, Pfizer, 2, 5, 6, Sandoz, 2, 6, Sanofi, 2, 5, 6, Teva, 2, 6, UCB, 2, 5, 6, UCBA, 5; P. Wickersham: Abbvie, 2, 6, Amgen, 6, AstraZeneca, 2, 6, Eli Lilly, 2, 5, 6, Novartis, 6; S. Liu-Leage: Eli Lilly, 3, 11; T. Panni: Eli Lilly, 3; R. Bolce: Eli Lilly and Company, 3, 11; B. Janos: Eli Lilly, 3, 11; M. Nissen: AbbVie/Abbott, 2, Eli Lilly, 2, 12, Involved in Clinical Trial, Janssen, 2, Novartis, 6, 12, research funding paid to institution, Pfizer, 6, UCB, 2, 12, funding support to attend EULAR 2023, paid to institution; J. Wei: Abbvie, 2, 5, 6, Amgen, 5, AstraZeneca, 6, BMS, 2, 5, 6, Celgene, 2, Chugai, 2, 6, Eisai, 2, 6, Eli Lilly, 2, 5, 6, Gilead, 5, GSK, 2, 5, Janssen, 2, 5, 6, Novartis, 2, 5, Pfizer, 2, 5, 6, Sanofi-Aventis, 2, SUN pharma, 5, TSH Taiwan, 2, UCB pharma, 2, 5.
Background/Purpose: The ASAS-EULAR recommendations for the management of axial spondyloarthritis (axSpA) advise that a patient be assessed for biological disease-modifying anti-rheumatic drug (bDMARD) treatment response after at least 12 weeks of treatment1. The recommended treatment target for axSpA is inactive disease (ID) or low disease activity (LDA)2, as together defined by an Ankylosing Spondylitis Disease Activity Score (ASDAS) <2.13. This analysis aimed to explore the association between treatment response at Week (W)12 and W24, and attainment of the ASDAS < 2.1 treat-to-target (T2T) recommendation at W52 in patients with radiographic (r-) axSpA treated with ixekizumab (IXE).
Methods: This post hoc analysis included patients randomly assigned to IXE 80mg every 4 weeks (N=81) from COAST-V (NCT02696785), a Phase 3 trial that investigated the efficacy of IXE in bDMARD-naïve patients with r-axSpA. The proportion of patients who achieved the ASDAS < 2.1 target response at W52 was evaluated among those in ID, LDA, high disease activity (HDA) or very high disease activity (VHDA) at W12 or W24. The proportion of patients who achieved ASDAS< 2.1 at W52 was also measured among those who attained meaningful clinical response at W12 or W24, as defined by a clinically important (CII, ∆ASDAS ≥1.1) or a major improvement (MI, ∆ASDAS ≥2.0) in ASDAS, improvement of ≥50% in Bath Ankylosing Spondylitis Disease Activity Index score (BASDAI50), or achievement of BASDAI < 4. Patient and disease characteristics are described according to responses at W12 or W24 and W52. Non-responder imputation was used to handle missing data.
Results: Of the 81 patients, 34 (42%) were in ID or LDA at W12 (8 ID, 26 LDA) and most of these patients met the ASDAS < 2.1 target at W52 (ID=100%, LDA=85%; Figure 1A). 47 patients achieved ASDAS CII at W12 and of those, 33 (70%) were in ID or LDA at W52 (Figure 1B). Similarly, most patients who met the ASDAS MI, BASDAI50 or BASDAI< 4 clinical response measures at W12 met ASDAS < 2.1 at W52 (81–94%; Figure 1B). 37 patients (46%) were in ID or LDA at W24 (13 ID, 24 LDA) and of those, 29 (78%) had already achieved ASDAS < 2.1 at W12 (Table 1), and most also met ASDAS < 2.1 at W52 (ID=100%, LDA=79%; Figure 1A). 52 patients achieved ASDAS CII at W24 and among them, 37 (71%) were in ID or LDA at W52 (Figure 1B). Most patients who met the ASDAS MI, BASDAI50 or BASDAI< 4 clinical response measures at W24 met ASDAS < 2.1 at W52 (80–89%; Figure 1B). Patients who did not meet ASDAS < 2.1 at W12 were more likely to be older, female, or with a longer symptom duration versus those who did, and 8 of these 47 patients (17%) subsequently achieved ASDAS < 2.1 at W24 (Table 1). 39 patients were in HDA or VHDA (ASDAS ≥2.1) at both W12 and W24 (Table 1), and 9 (23%) of these patients (11% of the 81 included patients) met the ASDAS < 2.1 target at W52.
Conclusion: These data regarding IXE-treated patients with r-axSpA reinforce the current ASAS-EULAR and T2T recommendations, in that those who achieved ASDAS CII or ASDAS < 2.1 at W12 and/or W24 were highly likely to attain the treatment target of ID or LDA at W52.
References
1. Ramiro SE et al. Ann Rheum Dis. 2023; 82(1):19-34
2. Smolen JS et al. Ann Rheum Dis. 2018; 77(1): 3-17
3. Machado PM et al. Ann Rheum Dis. 2018; 77(10): 1539-1540


S. Ramiro: AbbVie, 2, 5, Eli Lilly, 2, Galapagos, 5, MSD, 2, 5, Novartis, 2, 5, Pfizer, 2, 5, Sanofi, 2, UCB Pharma, 2, 5; C. Lukas: Abbvie, 2, 6, Amgen, 2, 6, Biogen, 2, 6, BMS, 2, 6, Eli Lilly, 2, 6, Janssen, 2, 6, MSD, 2, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche Chugai, 2, 6, UCB, 2, 6; L. Bessette: AbbVie, 2, 5, 6, Amgen, 2, 5, 6, Bristol Myers Squibb, 2, 5, 6, Eli Lilly, 2, 5, 6, Fresenius Kabi, 2, 6, Gilead, 2, 5, 6, JAMP Pharma, 2, 5, 6, Janssen, 2, 5, 6, Novartis, 2, 5, 6, Organon, 2, 6, Pfizer, 2, 5, 6, Sandoz, 2, 6, Sanofi, 2, 5, 6, Teva, 2, 6, UCB, 2, 5, 6, UCBA, 5; P. Wickersham: Abbvie, 2, 6, Amgen, 6, AstraZeneca, 2, 6, Eli Lilly, 2, 5, 6, Novartis, 6; S. Liu-Leage: Eli Lilly, 3, 11; T. Panni: Eli Lilly, 3; R. Bolce: Eli Lilly and Company, 3, 11; B. Janos: Eli Lilly, 3, 11; M. Nissen: AbbVie/Abbott, 2, Eli Lilly, 2, 12, Involved in Clinical Trial, Janssen, 2, Novartis, 6, 12, research funding paid to institution, Pfizer, 6, UCB, 2, 12, funding support to attend EULAR 2023, paid to institution; J. Wei: Abbvie, 2, 5, 6, Amgen, 5, AstraZeneca, 6, BMS, 2, 5, 6, Celgene, 2, Chugai, 2, 6, Eisai, 2, 6, Eli Lilly, 2, 5, 6, Gilead, 5, GSK, 2, 5, Janssen, 2, 5, 6, Novartis, 2, 5, Pfizer, 2, 5, 6, Sanofi-Aventis, 2, SUN pharma, 5, TSH Taiwan, 2, UCB pharma, 2, 5.



