Poster Session B
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (1412–1441) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II: SpA
1418: Efficacy of the Oral, Selective, Allosteric Tyrosine Kinase 2 Inhibitor, Deucravacitinib, on Psoriasis in Patients with Active PsA: Results from a Phase 2 Trial
Monday, November 13, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- MN
Miroslawa Nowak, MD
Bristol Myers Squibb
Princeton, NJ, United StatesDisclosure information not submitted.
Abstract Poster Presenter(s)
Alice B. Gottlieb1, April W. Armstrong2, Joseph Merola3, Andrew Napoli4, Miroslawa Nowak4, Subhashis Banerjee4, Thomas Lehman5 and Philip J. Mease6, 1Icahn School of Medicine at Mount Sinai, New York, NY, 2Keck School of Medicine of University of Southern California, Los Angeles, CA, 3Harvard Medical School, Brigham and Women's Hospital, Newton, MA, 4Bristol Myers Squibb, Princeton, NJ, 5Bristol Myers Squibb, Philadelphia, PA, 6Swedish Medical Center/Providence St. Joseph Health and University of Washington School of Medicine, Seattle, WA
Background/Purpose: Tyrosine kinase 2 (TYK2) mediates signaling of key cytokines involved in plaque psoriasis (PsO) and PsA pathophysiology. Deucravacitinib is a first-in-class, oral, selective, allosteric TYK2 inhibitor approved in multiple countries for the treatment of adults with moderate to severe PsO, based on superiority of deucravacitinib over apremilast and placebo (PBO) in various outcome measures in 4 phase 3 trials, including in China and Japan (NCT03611751, NCT03624127, NCT04167462, NCT03924427) in patients with moderate to severe PsO.1,2 DEUC also improved multiple measures of disease activity, including in arthritis and PsO, vs PBO in a phase 2 trial (NCT03881059) in patients with active PsA.3 This analysis further evaluated the efficacy ofdeucravacitinib on PsO in patients with concomitant PsA in the phase 2 trial.
Methods: The phase 2 double-blind trial in PsA randomized patients (N=203) 1:1:1 to PBO, deucravacitinib 6 mg once daily (QD), or deucravacitinib 12 mg QD. After wk 16 (part A), patients could optionally enroll in a double-blind period until wk 52 (part B), where deucravacitinib-treated patients with minimal disease activity at wk 16 continued deucravacitinib treatment to wk 52. PsO disease activity measures, including mean body surface area (BSA), mean Psoriasis Area and Severity Index (PASI) score, and achievement of different treat-to-target PASI and BSA thresholds, were assessed at week 16 in part A.
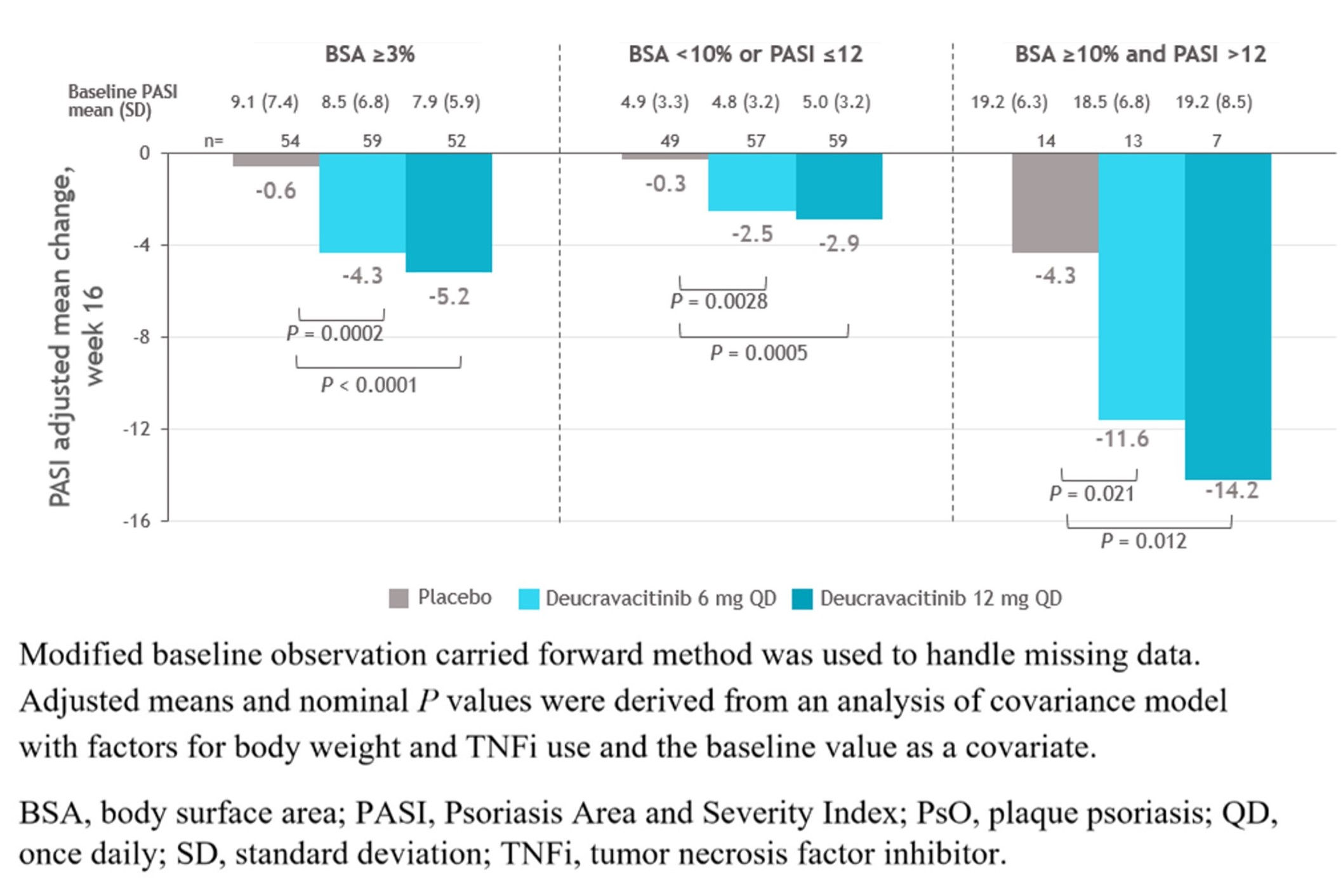
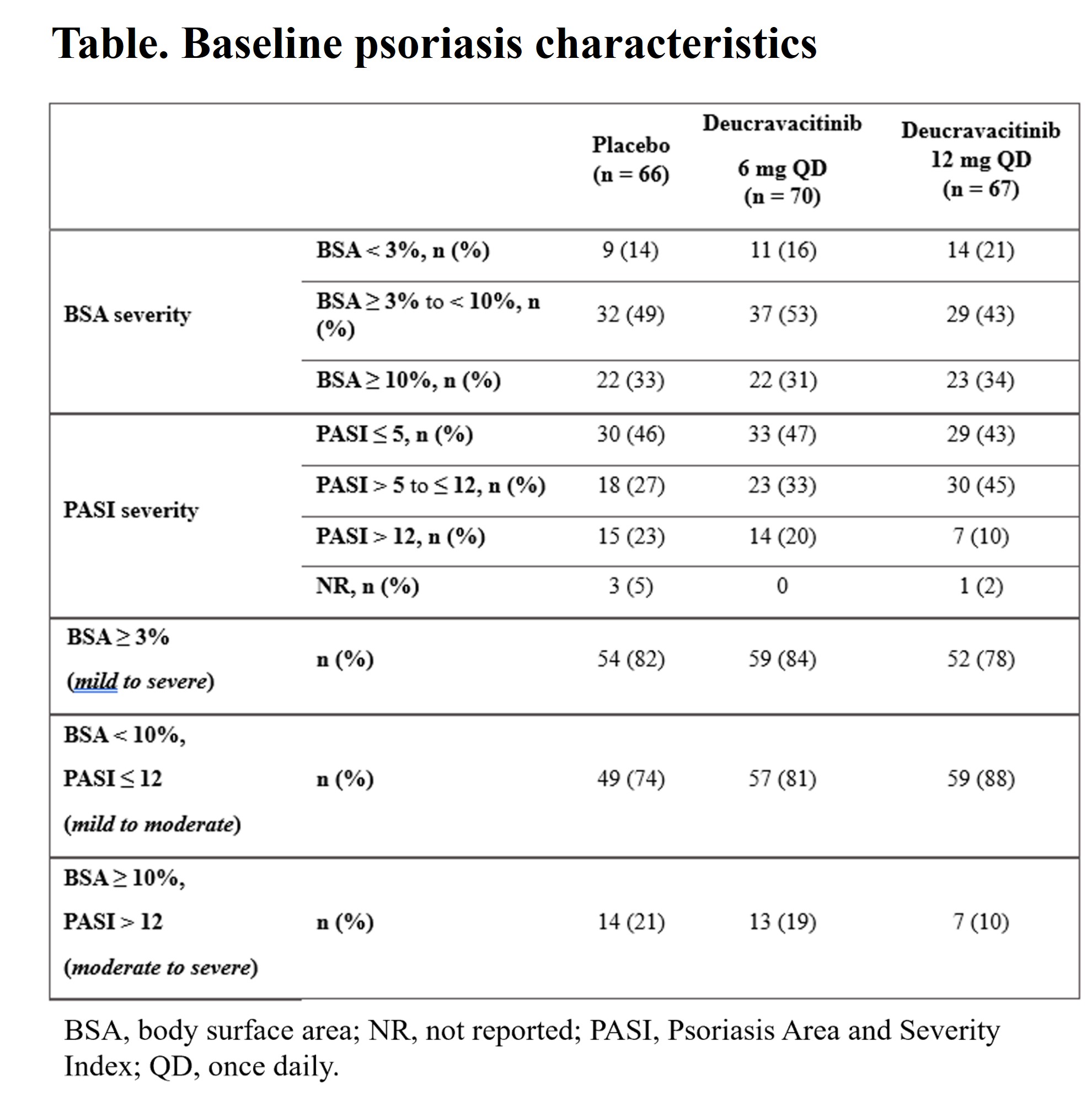
Results: At baseline (BL), PsO characteristics were generally similar across treatment groups, with most evaluable patients (≥ 74%) having a PASI< 12 or BSA< 10 (Table). At wk 16, mean PASI scores significantly decreased from BL and the improvements were significantly greater with deucravacitinib in PBO in patients treated with deucravacitinib vs PBO, even in those with very low BL PASI scores (Figure). Deucravacitinib treatment significantly decreased PASI scores from BL in patients with 6 mg QD and 12 mg QD vs PBO, respectively inpatients on background conventional synthetic (cs) DMARD (−4.0 and −4.9 vs −2.3; P < 0.05 for both) as well as those without csDMARD use (−3.7 and −4.0 vs +2.5; P < 0.001, for both) vs PBO. At wk 16, a greater proportion of patients treated with deucravacitinib at either dose vs PBO achieved PASI ≤ 1 in patients with mild to moderate or moderate to severe PsO. Decreases in mean PASI score at wk 16 were maintained through wk 52 in patients who achieved MDA and continued treatment with deucravacitinib 6 mg or 12 mg QD in part B of the study.
Conclusion: Treatment with deucravacitinib significantly improved PsO in patients with PsA, regardless of BL PsO severity and background csDMARD use. Of note, improvement in the moderate to severe PsO patient subgroup was comparable with that observed in the phase 3 POETYK PSO-1 trial in patients with moderate to severe PsO.1
References:
1. Armstrong A, et al. J Am Acad Dermatol 2023;88:29–39.
2. Strober B, et al. J Am Acad Dermatol 2023;88:40–51.
3. Mease PJ, et al. Ann Rheum Dis 2022;81:815–822.
A. Gottlieb: Amgen, 1, 2, AnaptysBio, 1, 2, 5, Avotres Therapeutics, 1, 2, Boehringer Ingelheim, 1, 2, Bristol Myers Squibb, 1, 2, 5, Dice Therapeutics, 1, 2, Eli Lilly, 1, 2, Janssen, 1, 2, MoonLake Immunotherapeutics, 5, Novartis, 1, 2, 5, Sanofi, 1, 2, UCB Pharma, 1, 2, 5, XBiotech, 1, 2; A. Armstrong: AbbVie, 5, Almirall, 1, 6, Arcutis, 1, 6, Aslan Pharmaceuticals, 1, 1, 6, 6, Beiersdorf, 1, 6, Boehringer Ingelheim/Parexel, 12, Personal fees, Bristol-Myers Squibb(BMS), 5, Celgene, 12, Personal fees, Dermavant, 1, 6, 12, Personal fees, Dermira, 1, 5, 6, Eli Lilly, 5, EPI Health, 1, 6, Genentech, 12, Personal fees, GSK, 12, Personal fees, Incyte, 1, 6, Janssen, 5, Kyowa Kirin, 5, Leo Pharma, 5, Lilly, 1, 6, Menlo Therapeutics, 12, Personal fees, Merck, 12, Personal fees, Mindera Health, 1, 6, Modernizing Medicine, 12, Personal fees, Nimbus, 1, 6, Novartis, 5, Ortho Dermatologics, 12, Personal fees, Pfizer, 12, Personal fees, Regeneron, 12, Personal fees, Sanofi Genzyme, 12, Personal fees, Science 37, 12, Personal fees, Sun, 1, 6, Sun Pharma, 12, Personal fees, UCB, 5, Valeant, 12, Personal fees; J. Merola: Abbvie, 2, 12, Investigator, Amgen, 2, 12, Investigator, Biogen, 2, 12, Investigator, Bristol-Myers Squibb, 2, 12, Investigator, Dermavant, 2, 12, Investigator, Eli Lilly, 2, 6, 12, Investigator, Janssen, 2, 12, Investigator, Leo Pharma, 2, 12, Investigator, Novartis, 2, 12, Investigator, Pfizer, 2, 12, Investigator, Regeneron, 2, 12, Investigator, Sanofi, 2, 12, Investigator, Sun Pharma, 2, 12, Investigator, UCB Pharma, 2, 12, Investigator; A. Napoli: Bristol-Myers Squibb(BMS), 3, 11; M. Nowak: Bristol-Myers Squibb(BMS), 3, 11; S. Banerjee: Bristol-Myers Squibb(BMS), 3, 11; T. Lehman: Bristol-Myers Squibb(BMS), 3; P. Mease: AbbVie, 2, 5, 6, Acelyrin, 2, Aclaris, 2, Amgen, 2, 5, 6, Boehringer Ingelheim, 2, Bristol Myers Squibb, 2, 5, Eli Lilly, 2, 5, 6, Galapagos, 2, Gilead, 2, GlaxoSmithKline, 2, Inmagene, 2, Janssen, 2, 5, 6, MoonLake Pharma, 2, Novartis, 2, 5, 6, Pfizer, 2, 5, 6, Sun Pharma, 2, 5, UCB Pharma, 2, 5, 6, Ventyx, 2, Xinthera, 2.
Background/Purpose: Tyrosine kinase 2 (TYK2) mediates signaling of key cytokines involved in plaque psoriasis (PsO) and PsA pathophysiology. Deucravacitinib is a first-in-class, oral, selective, allosteric TYK2 inhibitor approved in multiple countries for the treatment of adults with moderate to severe PsO, based on superiority of deucravacitinib over apremilast and placebo (PBO) in various outcome measures in 4 phase 3 trials, including in China and Japan (NCT03611751, NCT03624127, NCT04167462, NCT03924427) in patients with moderate to severe PsO.1,2 DEUC also improved multiple measures of disease activity, including in arthritis and PsO, vs PBO in a phase 2 trial (NCT03881059) in patients with active PsA.3 This analysis further evaluated the efficacy ofdeucravacitinib on PsO in patients with concomitant PsA in the phase 2 trial.
Methods: The phase 2 double-blind trial in PsA randomized patients (N=203) 1:1:1 to PBO, deucravacitinib 6 mg once daily (QD), or deucravacitinib 12 mg QD. After wk 16 (part A), patients could optionally enroll in a double-blind period until wk 52 (part B), where deucravacitinib-treated patients with minimal disease activity at wk 16 continued deucravacitinib treatment to wk 52. PsO disease activity measures, including mean body surface area (BSA), mean Psoriasis Area and Severity Index (PASI) score, and achievement of different treat-to-target PASI and BSA thresholds, were assessed at week 16 in part A.
Results: At baseline (BL), PsO characteristics were generally similar across treatment groups, with most evaluable patients (≥ 74%) having a PASI< 12 or BSA< 10 (Table). At wk 16, mean PASI scores significantly decreased from BL and the improvements were significantly greater with deucravacitinib in PBO in patients treated with deucravacitinib vs PBO, even in those with very low BL PASI scores (Figure). Deucravacitinib treatment significantly decreased PASI scores from BL in patients with 6 mg QD and 12 mg QD vs PBO, respectively inpatients on background conventional synthetic (cs) DMARD (−4.0 and −4.9 vs −2.3; P < 0.05 for both) as well as those without csDMARD use (−3.7 and −4.0 vs +2.5; P < 0.001, for both) vs PBO. At wk 16, a greater proportion of patients treated with deucravacitinib at either dose vs PBO achieved PASI ≤ 1 in patients with mild to moderate or moderate to severe PsO. Decreases in mean PASI score at wk 16 were maintained through wk 52 in patients who achieved MDA and continued treatment with deucravacitinib 6 mg or 12 mg QD in part B of the study.
Conclusion: Treatment with deucravacitinib significantly improved PsO in patients with PsA, regardless of BL PsO severity and background csDMARD use. Of note, improvement in the moderate to severe PsO patient subgroup was comparable with that observed in the phase 3 POETYK PSO-1 trial in patients with moderate to severe PsO.1
References:
1. Armstrong A, et al. J Am Acad Dermatol 2023;88:29–39.
2. Strober B, et al. J Am Acad Dermatol 2023;88:40–51.
3. Mease PJ, et al. Ann Rheum Dis 2022;81:815–822.

Figure. PASI mean change from baseline at week 16 by psoriasis severity at baseline

Table. Baseline psoriasis characteristics
A. Gottlieb: Amgen, 1, 2, AnaptysBio, 1, 2, 5, Avotres Therapeutics, 1, 2, Boehringer Ingelheim, 1, 2, Bristol Myers Squibb, 1, 2, 5, Dice Therapeutics, 1, 2, Eli Lilly, 1, 2, Janssen, 1, 2, MoonLake Immunotherapeutics, 5, Novartis, 1, 2, 5, Sanofi, 1, 2, UCB Pharma, 1, 2, 5, XBiotech, 1, 2; A. Armstrong: AbbVie, 5, Almirall, 1, 6, Arcutis, 1, 6, Aslan Pharmaceuticals, 1, 1, 6, 6, Beiersdorf, 1, 6, Boehringer Ingelheim/Parexel, 12, Personal fees, Bristol-Myers Squibb(BMS), 5, Celgene, 12, Personal fees, Dermavant, 1, 6, 12, Personal fees, Dermira, 1, 5, 6, Eli Lilly, 5, EPI Health, 1, 6, Genentech, 12, Personal fees, GSK, 12, Personal fees, Incyte, 1, 6, Janssen, 5, Kyowa Kirin, 5, Leo Pharma, 5, Lilly, 1, 6, Menlo Therapeutics, 12, Personal fees, Merck, 12, Personal fees, Mindera Health, 1, 6, Modernizing Medicine, 12, Personal fees, Nimbus, 1, 6, Novartis, 5, Ortho Dermatologics, 12, Personal fees, Pfizer, 12, Personal fees, Regeneron, 12, Personal fees, Sanofi Genzyme, 12, Personal fees, Science 37, 12, Personal fees, Sun, 1, 6, Sun Pharma, 12, Personal fees, UCB, 5, Valeant, 12, Personal fees; J. Merola: Abbvie, 2, 12, Investigator, Amgen, 2, 12, Investigator, Biogen, 2, 12, Investigator, Bristol-Myers Squibb, 2, 12, Investigator, Dermavant, 2, 12, Investigator, Eli Lilly, 2, 6, 12, Investigator, Janssen, 2, 12, Investigator, Leo Pharma, 2, 12, Investigator, Novartis, 2, 12, Investigator, Pfizer, 2, 12, Investigator, Regeneron, 2, 12, Investigator, Sanofi, 2, 12, Investigator, Sun Pharma, 2, 12, Investigator, UCB Pharma, 2, 12, Investigator; A. Napoli: Bristol-Myers Squibb(BMS), 3, 11; M. Nowak: Bristol-Myers Squibb(BMS), 3, 11; S. Banerjee: Bristol-Myers Squibb(BMS), 3, 11; T. Lehman: Bristol-Myers Squibb(BMS), 3; P. Mease: AbbVie, 2, 5, 6, Acelyrin, 2, Aclaris, 2, Amgen, 2, 5, 6, Boehringer Ingelheim, 2, Bristol Myers Squibb, 2, 5, Eli Lilly, 2, 5, 6, Galapagos, 2, Gilead, 2, GlaxoSmithKline, 2, Inmagene, 2, Janssen, 2, 5, 6, MoonLake Pharma, 2, Novartis, 2, 5, 6, Pfizer, 2, 5, 6, Sun Pharma, 2, 5, UCB Pharma, 2, 5, 6, Ventyx, 2, Xinthera, 2.



