Poster Session A
Periodic fever syndromes, autoinflammatory diseases, Still’s disease and MAS/HLH
Session: (0252–0282) Miscellaneous Rheumatic & Inflammatory Diseases Poster I
0280: Immunosuppression in the Treatment of Susac Syndrome: A Retrospective Evaluation of the Largest Cohort of Susac Syndrome
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- AB
Adam Brown, MD, BS
Cleveland Clinic
Shaker Heights, OH, United StatesDisclosure(s): Amgen: Consultant (Ongoing)
Abstract Poster Presenter(s)
Adam Brown1, Robert Rennebohm2, Ghulam Abbas Kharal2, Devon Conway2, Sunil Srivastava3, Sumit Sharma2, Careen Lowder2, Leonard Calabrese4 and Rula Hajj-Ali2, 1Cleveland Clinic Department of Rheumatic and Immunologic Diseases, Cleveland, OH, 2Cleveland Clinic, Cleveland, OH, 3Cincinnati Eye Institute, Cincinnati, OH, 4Cleveland Clinic / Department of Rheumatic and Immunologic Diseases, Cleveland, OH
Background/Purpose: Susac Syndrome is a rare autoimmune condition causing microvascular occlusions in the brain, retina and inner ear leading to the characteristic triad of encephalopathy, branch retinal artery occlusion (BRAO) and sensorineural hearing loss potentially leading to permanent disability and death if untreated. Approximately 400 cases of Susac Syndrome have been reported since its initial description in the 1970s. Most cases are from small case series utilizing various combinations of immunosuppression with variable outcomes. It is unclear what immunosuppressive regimen is most likely to result in reduced risk of disease flares. The goal of this study is to retrospectively evaluate all Susac Cases in our institution and compare immunosuppressive regimens.
Methods: A retrospective chart review of 134 patients with Susac Syndrome ICD diagnosis code within our institute's electronic medical record system was performed. The following were recorded: patient demographics, presenting signs/symptoms, whether the full triad of encephalopathy, vision and hearing loss were present, as well as immunosuppressive medications initiated, length of follow up and whether a patient had a flare while on immunosuppression. Flares were defined by objective changes in brain MRI, fluorescein angiography or audiometry in addition to clinical symptoms.
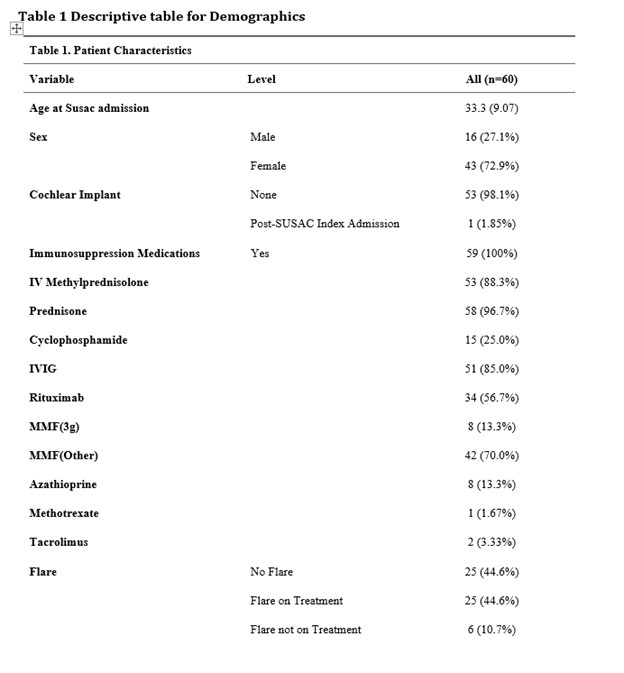
Results: Sixty patients met the European Susac Consortium criteria. The triad of encephalopathy with MRI changes of the corpus callosum, BRAO and hearing loss documented with audiometry were present in 65% of cases. The average age at presentation was 33 years of age and females accounted for 73% of patients. Patients received the following immunosuppressive medications: glucocorticoids 100%, IVIG (85%), mycophenolate mofetil MMF (83%) most patients receiving less than 3 grams daily (84%). Rituximab was given to 56% of patients and cyclophosphamide was given in 25% of cases. Azathioprine, methotrexate or tacrolimus together made up 18% of cases.
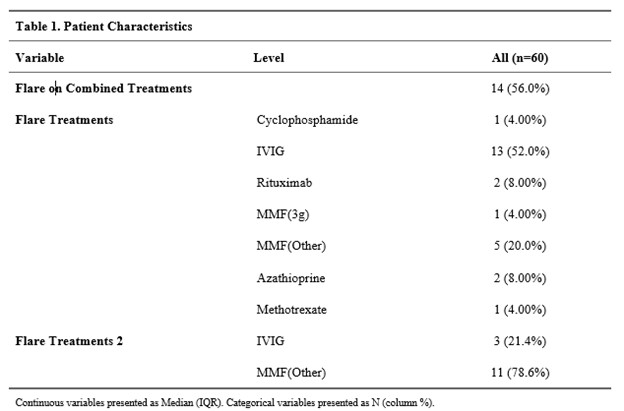
Flares were documented in 51% of patients after the initial diagnosis of Susac Syndrome, occurring on average 24 months after disease onset. 74% of patients were on medications at the time of the flare. Flares occurred on the following therapies: IVIG 16 (32%), MMF (< 3 grams) 16 (38%), MMF (3 grams) 1 (12%), Rituximab 2 (5%) and cyclophosphamide 1 (6%).
MMF and Rituximab were two of the more commonly used therapeutics, often in combination with IVIG. MMF overall, had a higher likelihood of experiencing flares when compared to patients on Rituximab (34% vs 5%) (P=0.003). This difference was most pronounced when compared to those on doses of MMF less than 3 grams daily (P < 0.001). In our cohort, a patient on MMF (all doses) is 5.8 times more likely to experience a flare compared to patients on Rituximab.
Conclusion: This is the largest cohort of patients suffering from Susac Syndrome. Based upon this single center retrospective cohort analysis, we conclude flares of disease were common over the first two years of follow up. These preliminary data suggest Rituximab may be superior to MMF in maintaining remission. Further study of this rare disease, including prospective controlled trials are needed.
A. Brown: Amgen, 2; R. Rennebohm: None; G. Kharal: None; D. Conway: Arena, 2, Biogen, 5, EMD Serono, 5, Horizon, 5, Novartis, 2; S. Srivastava: AbbVie/Abbott, 2, Allergan, 5, Bausch and Lomb, 2, Eyepoint, 2, 5, Eyevensys, 2, 5, Novartis, 2, Regeneron, 2, 5, Santen, 5, Zeiss, 2; S. Sharma: AbbVie/Abbott, 2, Alimera, 2, Allergan, 2, Apellis, 2, Bausch and Lomb, 2, Clearside, 2, Eyepoint, 2, Genentech, 2, 5, Gilead, 5, Regeneron, 2, RegenxBio, 2, Roche, 2, 5; C. Lowder: None; L. Calabrese: AstraZeneca, 6, Bristol-Myers Squibb(BMS), 2, Galvani, 2, Genentech, 2, GlaxoSmithKlein(GSK), 2, sanofi, 2, 6; R. Hajj-Ali: Amgen, 2, GlaxoSmithKlein(GSK), 2, uptodate, 9.
Background/Purpose: Susac Syndrome is a rare autoimmune condition causing microvascular occlusions in the brain, retina and inner ear leading to the characteristic triad of encephalopathy, branch retinal artery occlusion (BRAO) and sensorineural hearing loss potentially leading to permanent disability and death if untreated. Approximately 400 cases of Susac Syndrome have been reported since its initial description in the 1970s. Most cases are from small case series utilizing various combinations of immunosuppression with variable outcomes. It is unclear what immunosuppressive regimen is most likely to result in reduced risk of disease flares. The goal of this study is to retrospectively evaluate all Susac Cases in our institution and compare immunosuppressive regimens.
Methods: A retrospective chart review of 134 patients with Susac Syndrome ICD diagnosis code within our institute's electronic medical record system was performed. The following were recorded: patient demographics, presenting signs/symptoms, whether the full triad of encephalopathy, vision and hearing loss were present, as well as immunosuppressive medications initiated, length of follow up and whether a patient had a flare while on immunosuppression. Flares were defined by objective changes in brain MRI, fluorescein angiography or audiometry in addition to clinical symptoms.
Results: Sixty patients met the European Susac Consortium criteria. The triad of encephalopathy with MRI changes of the corpus callosum, BRAO and hearing loss documented with audiometry were present in 65% of cases. The average age at presentation was 33 years of age and females accounted for 73% of patients. Patients received the following immunosuppressive medications: glucocorticoids 100%, IVIG (85%), mycophenolate mofetil MMF (83%) most patients receiving less than 3 grams daily (84%). Rituximab was given to 56% of patients and cyclophosphamide was given in 25% of cases. Azathioprine, methotrexate or tacrolimus together made up 18% of cases.
Flares were documented in 51% of patients after the initial diagnosis of Susac Syndrome, occurring on average 24 months after disease onset. 74% of patients were on medications at the time of the flare. Flares occurred on the following therapies: IVIG 16 (32%), MMF (< 3 grams) 16 (38%), MMF (3 grams) 1 (12%), Rituximab 2 (5%) and cyclophosphamide 1 (6%).
MMF and Rituximab were two of the more commonly used therapeutics, often in combination with IVIG. MMF overall, had a higher likelihood of experiencing flares when compared to patients on Rituximab (34% vs 5%) (P=0.003). This difference was most pronounced when compared to those on doses of MMF less than 3 grams daily (P < 0.001). In our cohort, a patient on MMF (all doses) is 5.8 times more likely to experience a flare compared to patients on Rituximab.
Conclusion: This is the largest cohort of patients suffering from Susac Syndrome. Based upon this single center retrospective cohort analysis, we conclude flares of disease were common over the first two years of follow up. These preliminary data suggest Rituximab may be superior to MMF in maintaining remission. Further study of this rare disease, including prospective controlled trials are needed.

Patient Characteristics and Immunosuppresion

Rates of Flare with Immunosuppression
A. Brown: Amgen, 2; R. Rennebohm: None; G. Kharal: None; D. Conway: Arena, 2, Biogen, 5, EMD Serono, 5, Horizon, 5, Novartis, 2; S. Srivastava: AbbVie/Abbott, 2, Allergan, 5, Bausch and Lomb, 2, Eyepoint, 2, 5, Eyevensys, 2, 5, Novartis, 2, Regeneron, 2, 5, Santen, 5, Zeiss, 2; S. Sharma: AbbVie/Abbott, 2, Alimera, 2, Allergan, 2, Apellis, 2, Bausch and Lomb, 2, Clearside, 2, Eyepoint, 2, Genentech, 2, 5, Gilead, 5, Regeneron, 2, RegenxBio, 2, Roche, 2, 5; C. Lowder: None; L. Calabrese: AstraZeneca, 6, Bristol-Myers Squibb(BMS), 2, Galvani, 2, Genentech, 2, GlaxoSmithKlein(GSK), 2, sanofi, 2, 6; R. Hajj-Ali: Amgen, 2, GlaxoSmithKlein(GSK), 2, uptodate, 9.



