Poster Session A
Systemic lupus erythematosus (SLE)
Session: (0543–0581) SLE – Diagnosis, Manifestations, & Outcomes Poster I
0568: Comparison of Plasma Protein Profiles and Endothelial Function in Patients with Pediatric-Onset Systemic Lupus Erythematosus and Healthy Controls
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- LK
Liyoung Kim, MD
Boston Children's Hospital
Brookline, MA, United StatesDisclosure information not submitted.
Abstract Poster Presenter(s)
Liyoung Kim1, Gabrielle Alonzi1, Marina Barguil Macedo2, Pamela F. Weiss3, Jane Newburger4, Karen Costenbader5, Christian Lood2 and Joyce Chang1, 1Boston Children's Hospital, Boston, MA, 2University of Washington, Seattle, WA, 3Children's Hospital of Philadelphia, Philadelphia, PA, 4Boston Children's Hospital, Boston, MA, 5Brigham and Women's Hospital and Harvard Medical School, Boston, MA
Background/Purpose: Patients with pediatric-onset systemic lupus erythematosus (pSLE) have elevated cardiovascular (CV) risk associated with accelerated atherosclerosis that begins in childhood. Endothelial dysfunction may be one of the earliest atherosclerotic precursors, but the role of endothelial function assessment for CV risk stratification in pSLE is unclear. We compared non-invasive endothelial function testing and plasma protein profiles between patients with pSLE and controls and evaluated associations between protein expression and endothelial dysfunction.
Methods: Forty-four pSLE subjects, aged 9–21 years without active Raynaud's, were recruited from pediatric rheumatology clinics at two tertiary centers. We recruited 24 age and sex-matched controls without chronic conditions from siblings of rheumatology patients, dermatology clinic or primary care network. Demographic data, traditional CV risk factors and clinical disease activity scores were collected. Endothelial function, quantified as the log-transformed reactive hyperemia index (LnRHI), was measured by peripheral arterial tonometry (EndoPAT, Itamar Medical). We measured plasma markers of neutrophil (myeloperoxidase (MPO)-DNA, neutrophil elastase-DNA, and calprotectin) and endothelial activation (ICAM-1, VCAM-1) hypothesized to induce endothelial injury, as well as a 96-target proteomic proximity extension assay (CVD-II panel, Olinkâ). We used linear regression models and Mann-Whitney U tests to evaluate differences in LnRHI and plasma biomarkers, respectively. Spearman rank correlation coefficients (r) were used to evaluate clinical factors and biomarkers associated with LnRHI in pSLE subjects.
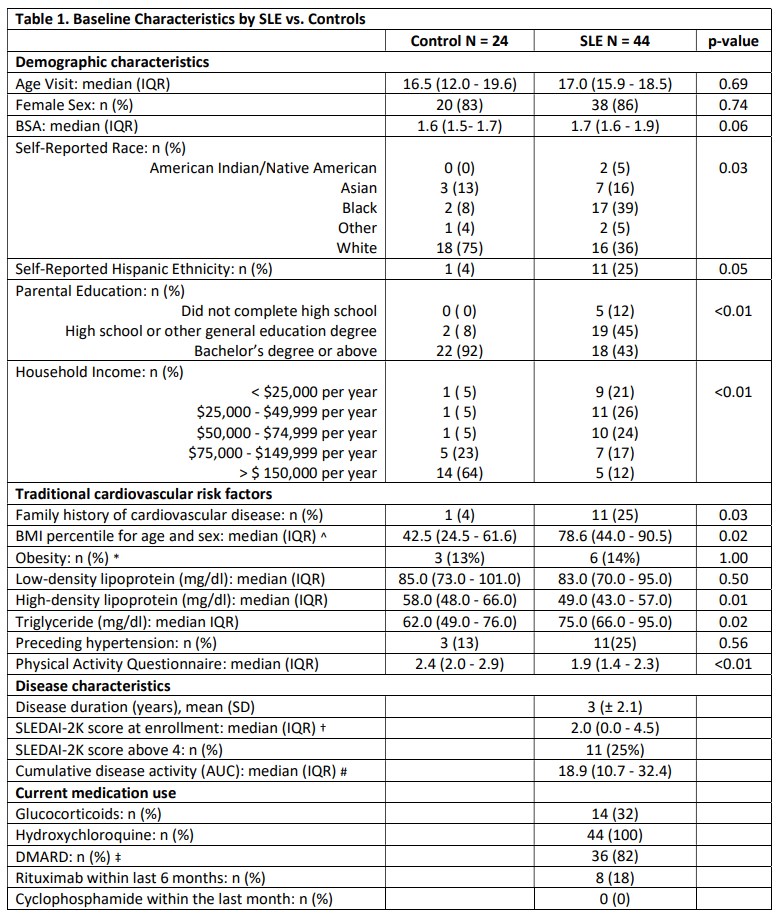
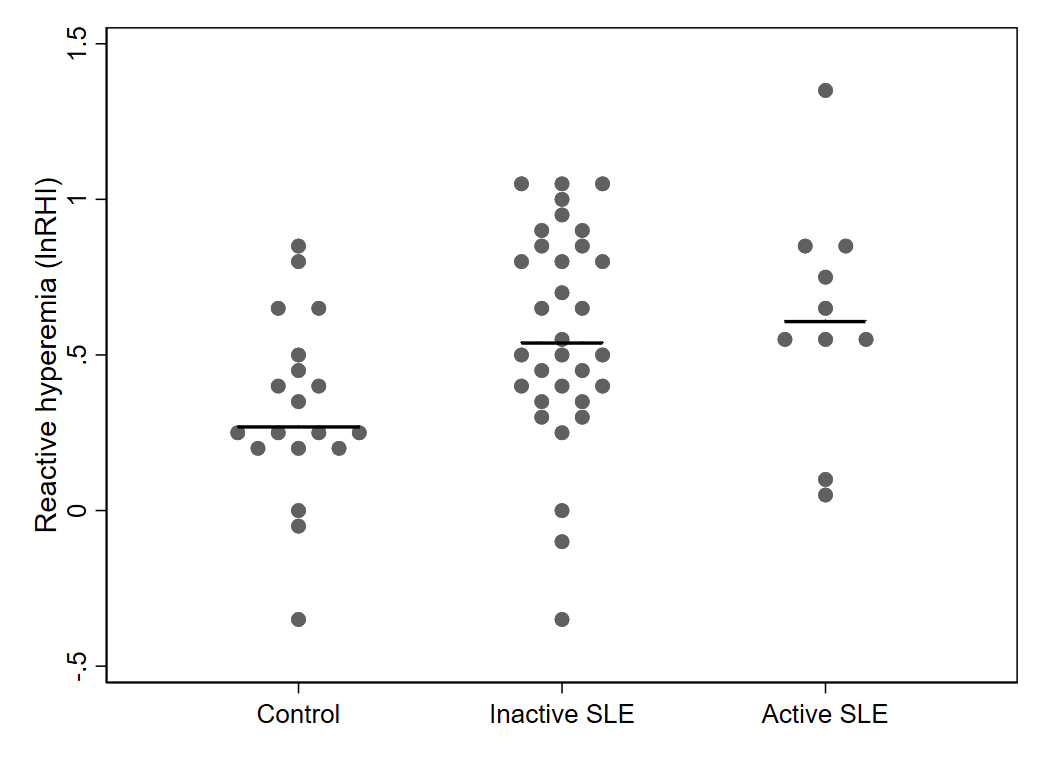
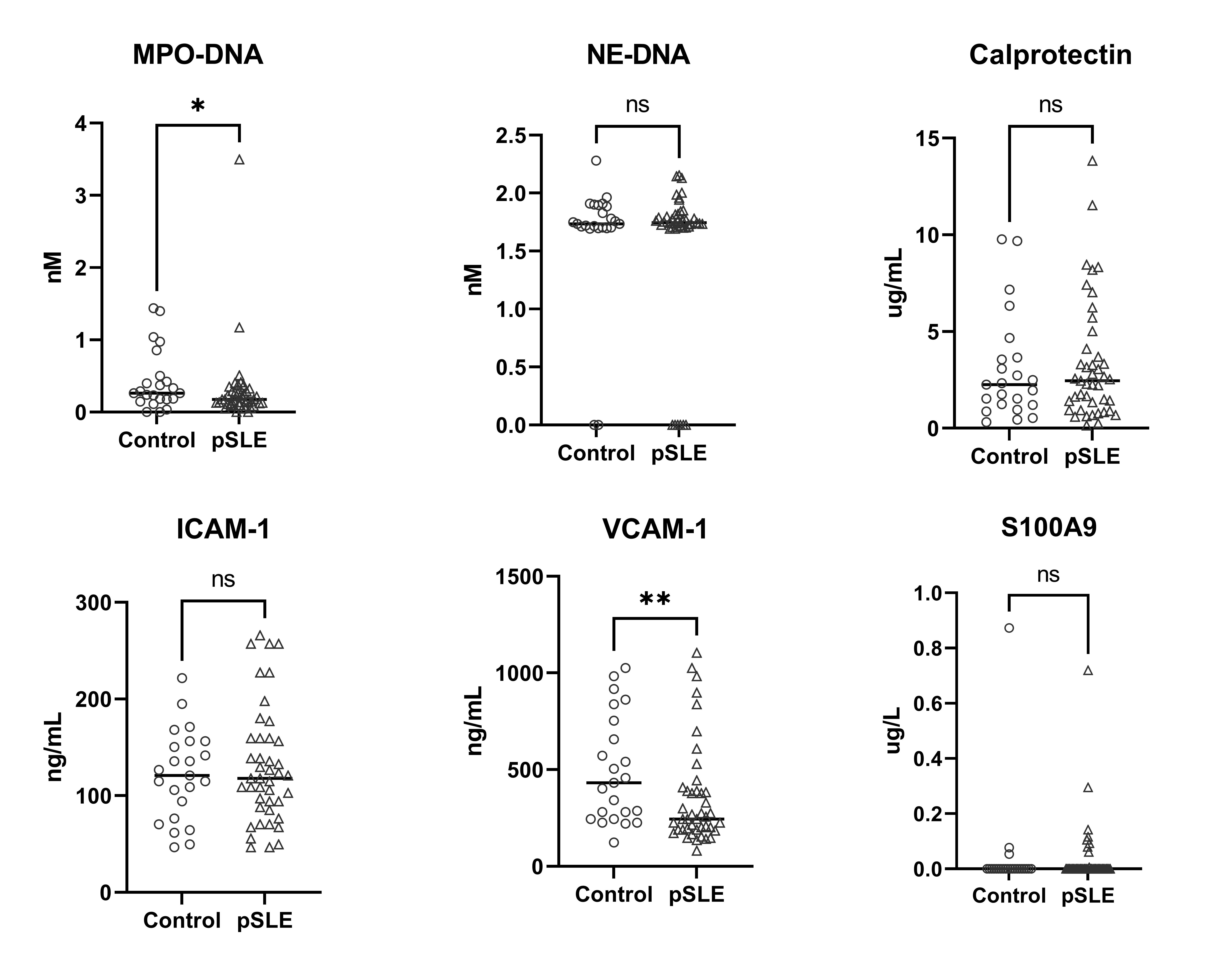
Results: Compared to controls, pSLE subjects were more likely to report Black race, lower parental education and lower household income (Table 1). pSLE subjects also had more CV risk factors, including family history of CVD, higher body mass index and triglyceride levels, lower high-density lipoprotein (HDL), and lower physical activity scores compared to controls. Of note, 75% of the pSLE cohort had low or inactive disease (SLEDAI-2K score≤ 4).Endothelial function testing revealed higher (better) average lnRHI in pSLE subjects vs. controls (b 0.26, p< 0.01), regardless of disease activity (Fig 1), and with adjustment for body surface area (BSA) (for finger size) and HDL (b 0.30, p< 0.01). There was no significant increase in neutrophil/endothelial activation markers or CVD-related proteins in subjects vs. controls. Conversely, median MPO-DNA and VCAM-1 levels were higher in controls (Fig 2). Only BSA and diastolic blood pressure were significantly associated with lnRHI (r 0.32, p=0.03, and r-0.32, p=0.04, respectively).
Conclusion: Our results unexpectedly demonstrated better endothelial function and lower MPO-DNA levels in children with pSLE compared to controls despite greater CV risk factors. This might be attributed to low SLE disease activity and/or effects of treatment. Further studies to understand how disease activity and immunomodulatory medications modify endothelial health in children with pSLE may provide insight into potential mechanisms of endothelial dysfunction and ideal disease activity targets.
L. Kim: None; G. Alonzi: None; M. Barguil Macedo: None; P. Weiss: Eli Lilly, 2, Novartis, 2, Pfizer, 2; J. Newburger: None; K. Costenbader: Amgen, 2, 5, AstraZeneca, 5, Bristol-Myers Squibb(BMS), 2, Cabaletta, 2, Eli Lilly, 2, Exagen Diagnostics, 5, Gilead, 5, GlaxoSmithKlein(GSK), 2, 5, Janssen, 2, 5; C. Lood: Amytryx, 5, Boehringer-Ingelheim, 5, Bristol-Myers Squibb(BMS), 5, Citryll, 2, Eli Lilly, 5, Gilead, 5, Horizon Therapeutics, 5, Pfizer, 5, Redd Pharma, 5, 11; J. Chang: None.
Background/Purpose: Patients with pediatric-onset systemic lupus erythematosus (pSLE) have elevated cardiovascular (CV) risk associated with accelerated atherosclerosis that begins in childhood. Endothelial dysfunction may be one of the earliest atherosclerotic precursors, but the role of endothelial function assessment for CV risk stratification in pSLE is unclear. We compared non-invasive endothelial function testing and plasma protein profiles between patients with pSLE and controls and evaluated associations between protein expression and endothelial dysfunction.
Methods: Forty-four pSLE subjects, aged 9–21 years without active Raynaud's, were recruited from pediatric rheumatology clinics at two tertiary centers. We recruited 24 age and sex-matched controls without chronic conditions from siblings of rheumatology patients, dermatology clinic or primary care network. Demographic data, traditional CV risk factors and clinical disease activity scores were collected. Endothelial function, quantified as the log-transformed reactive hyperemia index (LnRHI), was measured by peripheral arterial tonometry (EndoPAT, Itamar Medical). We measured plasma markers of neutrophil (myeloperoxidase (MPO)-DNA, neutrophil elastase-DNA, and calprotectin) and endothelial activation (ICAM-1, VCAM-1) hypothesized to induce endothelial injury, as well as a 96-target proteomic proximity extension assay (CVD-II panel, Olinkâ). We used linear regression models and Mann-Whitney U tests to evaluate differences in LnRHI and plasma biomarkers, respectively. Spearman rank correlation coefficients (r) were used to evaluate clinical factors and biomarkers associated with LnRHI in pSLE subjects.
Results: Compared to controls, pSLE subjects were more likely to report Black race, lower parental education and lower household income (Table 1). pSLE subjects also had more CV risk factors, including family history of CVD, higher body mass index and triglyceride levels, lower high-density lipoprotein (HDL), and lower physical activity scores compared to controls. Of note, 75% of the pSLE cohort had low or inactive disease (SLEDAI-2K score≤ 4).Endothelial function testing revealed higher (better) average lnRHI in pSLE subjects vs. controls (b 0.26, p< 0.01), regardless of disease activity (Fig 1), and with adjustment for body surface area (BSA) (for finger size) and HDL (b 0.30, p< 0.01). There was no significant increase in neutrophil/endothelial activation markers or CVD-related proteins in subjects vs. controls. Conversely, median MPO-DNA and VCAM-1 levels were higher in controls (Fig 2). Only BSA and diastolic blood pressure were significantly associated with lnRHI (r 0.32, p=0.03, and r-0.32, p=0.04, respectively).
Conclusion: Our results unexpectedly demonstrated better endothelial function and lower MPO-DNA levels in children with pSLE compared to controls despite greater CV risk factors. This might be attributed to low SLE disease activity and/or effects of treatment. Further studies to understand how disease activity and immunomodulatory medications modify endothelial health in children with pSLE may provide insight into potential mechanisms of endothelial dysfunction and ideal disease activity targets.

Fisher's exact or Mann-Whitney U tests were used to compare clinical characteristics between pSLE subjects and controls.
^ BMI-for-age-and-sex percentiles are used for ages 2-19 years.
* Obesity defined by BMI 95th percentile for subjects 2-19 years old, or BMI >30 kg/m2 in subjects 20 years or older.
†SLEDAI <5, low disease activity; 6–10, moderate; 11–19, high; maximum, 105
#The area under the curve (AUC) was calculated as a measure of long-term burden.
‡ Disease-modifying anti-rheumatic drugs (DMARDs) include mycophenolate mofetil, methotrexate, tacrolimus, and azathioprine.
^ BMI-for-age-and-sex percentiles are used for ages 2-19 years.
* Obesity defined by BMI 95th percentile for subjects 2-19 years old, or BMI >30 kg/m2 in subjects 20 years or older.
†SLEDAI <5, low disease activity; 6–10, moderate; 11–19, high; maximum, 105
#The area under the curve (AUC) was calculated as a measure of long-term burden.
‡ Disease-modifying anti-rheumatic drugs (DMARDs) include mycophenolate mofetil, methotrexate, tacrolimus, and azathioprine.

Mean differences in baseline reactive hyperemia (LnRHI) between 42 pSLE subjects and 19 controls with evaluable peripheral arterial tonometry tracings. Both subjects with low/inactive SLE (SLEDAI-2K≤ 4) and active SLE (SLEDAI-2K> 4) had statistically significant higher average LnRHI values compared to controls (p=0.01 and p< 0.01, respectively).

Selected plasma biomarkers of neutrophil extracellular traps (myeloperoxidase [MPO]-DNA complexes, neutrophil elastase [NE]-DNA, neutrophil activation (calprotectin, S100A9), or endothelial activation (VCAM-1, ICAM-1) in 43 pSLE subjects at enrollment compared to 23 controls with evaluable samples, using Mann-Whitney U tests. * p<0.05; ** p<0.01
L. Kim: None; G. Alonzi: None; M. Barguil Macedo: None; P. Weiss: Eli Lilly, 2, Novartis, 2, Pfizer, 2; J. Newburger: None; K. Costenbader: Amgen, 2, 5, AstraZeneca, 5, Bristol-Myers Squibb(BMS), 2, Cabaletta, 2, Eli Lilly, 2, Exagen Diagnostics, 5, Gilead, 5, GlaxoSmithKlein(GSK), 2, 5, Janssen, 2, 5; C. Lood: Amytryx, 5, Boehringer-Ingelheim, 5, Bristol-Myers Squibb(BMS), 5, Citryll, 2, Eli Lilly, 5, Gilead, 5, Horizon Therapeutics, 5, Pfizer, 5, Redd Pharma, 5, 11; J. Chang: None.



