Poster Session A
Systemic lupus erythematosus (SLE)
Session: (0543–0581) SLE – Diagnosis, Manifestations, & Outcomes Poster I
0566: Characterization of Disease Activity Using a Novel Physiologic Biomarker in Pediatric SLE
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- SS
Sangeeta Sule, MD, PhD (she/her/hers)
Children's National Hospital
Washington, DC, United StatesDisclosure information not submitted.
Abstract Poster Presenter(s)
Sangeeta Sule, Olivia Lamanna, Kevin Jackson, Sun-Young Ahn, Tineer Ahmed and Julia Finkel, Children's National Hospital, Washington, DC
Background/Purpose: Systemic lupus erythematosus (SLE) is a complex inflammatory disease involving many organ systems. To better understand and treat SLE, two phenotypes have been proposed. Type 1 is defined by signs of autoreactivity and inflammation such as nephritis, arthritis, and rash. The Type 2 state is thought to result from cytokine mediated peripheral and central (nociplastic) changes causing fatigue, sleep disturbance, and widespread pain, which often overlap with the manifestations of fibromyalgia (FM). While the grouping of patients based on symptoms and the clinical/serological components of disease is an established practice in the field, the application of this approach is limited by the tools available to assess and measure patient disease activity.
Methods: We performed phenotype testing on 12 patients (ages 13-21y) diagnosed with SLE being seen at the Children’s National Multidisciplinary Lupus Clinic. All patients met the ACR diagnostic criteria for SLE. This approach employs a novel medical device invented at Children’s National Hospital. This device is a novel integration of non-invasive and innocuous neuroselective electrical stimulation with infrared pupillometry (nPRD) to activate specific sensory nerve fiber types and allows for an objective quantified characterization of sensory nerve fiber activity. This technology measures the impact of SLE-associated inflammation on sensory nerve fiber activity. The relationship of the nPRD-derived AUC for each of the three sensory fibers (Aβ, C and Aδ) at a given time are compared using a ratio (e.g., [AUCAδ-AUCC]/AUCAβ ) to derive a quantitative index, which is the primary measurement endpoint. This index is used as the clinical output to contextualize the type and intensity of nociceptive processing. The data were analyzed using student’s t-tests and Pearson’s Correlation.
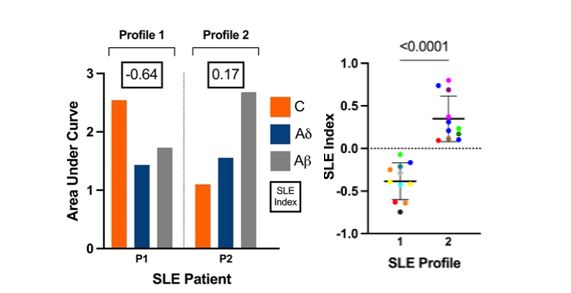
Results: Using our novel technology and method we observed two overarching and significantly different SLE physiologic phenotypes (p< 0.001, Figure 1). P1 has relatively increased C fiber sensitivity and decreased Aδ fiber sensitivity, which has been designated “profile 1”. P2 has a dominating Aβ fiber with less pronounced C and Aδ measures, which has been designated “profile 2”.Nociceptive profile 1 aligns with SLE Type 1 (active disease) whereas, nociceptive profile 2 aligns with SLE Type 2 (FM and/or nociplastic).
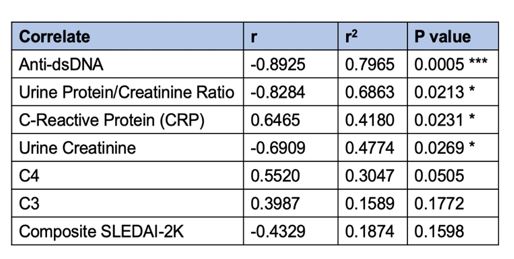
We then correlated our SLE Index to the SLEDAI-2K composite score and specific serological components, which are shown in Figure 2 and summarized in Table 1. These data demonstrate a statistically significant correlation to four parameters expected to be elevated or decreased in the SLE Type 1 active state.
Conclusion: Our preliminary data support the potential of our novel, objective, physiologic measure to characterize and quantify SLE disease activity. These findings will be verified in future studies.
.jpg)
S. Sule: None; O. Lamanna: None; K. Jackson: AlgometRx, Inc., 3, 4, 8; S. Ahn: None; T. Ahmed: None; J. Finkel: AlgometRx, 4, 10.
Background/Purpose: Systemic lupus erythematosus (SLE) is a complex inflammatory disease involving many organ systems. To better understand and treat SLE, two phenotypes have been proposed. Type 1 is defined by signs of autoreactivity and inflammation such as nephritis, arthritis, and rash. The Type 2 state is thought to result from cytokine mediated peripheral and central (nociplastic) changes causing fatigue, sleep disturbance, and widespread pain, which often overlap with the manifestations of fibromyalgia (FM). While the grouping of patients based on symptoms and the clinical/serological components of disease is an established practice in the field, the application of this approach is limited by the tools available to assess and measure patient disease activity.
Methods: We performed phenotype testing on 12 patients (ages 13-21y) diagnosed with SLE being seen at the Children’s National Multidisciplinary Lupus Clinic. All patients met the ACR diagnostic criteria for SLE. This approach employs a novel medical device invented at Children’s National Hospital. This device is a novel integration of non-invasive and innocuous neuroselective electrical stimulation with infrared pupillometry (nPRD) to activate specific sensory nerve fiber types and allows for an objective quantified characterization of sensory nerve fiber activity. This technology measures the impact of SLE-associated inflammation on sensory nerve fiber activity. The relationship of the nPRD-derived AUC for each of the three sensory fibers (Aβ, C and Aδ) at a given time are compared using a ratio (e.g., [AUCAδ-AUCC]/AUCAβ ) to derive a quantitative index, which is the primary measurement endpoint. This index is used as the clinical output to contextualize the type and intensity of nociceptive processing. The data were analyzed using student’s t-tests and Pearson’s Correlation.
Results: Using our novel technology and method we observed two overarching and significantly different SLE physiologic phenotypes (p< 0.001, Figure 1). P1 has relatively increased C fiber sensitivity and decreased Aδ fiber sensitivity, which has been designated “profile 1”. P2 has a dominating Aβ fiber with less pronounced C and Aδ measures, which has been designated “profile 2”.Nociceptive profile 1 aligns with SLE Type 1 (active disease) whereas, nociceptive profile 2 aligns with SLE Type 2 (FM and/or nociplastic).
We then correlated our SLE Index to the SLEDAI-2K composite score and specific serological components, which are shown in Figure 2 and summarized in Table 1. These data demonstrate a statistically significant correlation to four parameters expected to be elevated or decreased in the SLE Type 1 active state.
Conclusion: Our preliminary data support the potential of our novel, objective, physiologic measure to characterize and quantify SLE disease activity. These findings will be verified in future studies.

Figure 1. Distinct nociceptive profiles detected in SLE cohort by nPRD. (left) Showing nPRD-derived AUC measures of C, Ad, and Ab fibers and SLE Index scores of patients with SLE. Patients are categorized into “Profile 1” and “Profile 2” as indicated. (right) Showing the SLE Index measures for Profile 1 and Profile 2. Statistics: unpaired t-test, significance is as indicated.
.jpg)
Figure 2. Anti-dsDNA antibodies and urine protein/creatinine ratio correlate with SLE Index. Plot showing line of best-fit and 95% confidence band of simple linear regression of the SLE Index with anti-dsDNA antibody (n=10) and the urine protein/creatinine ratio (n=7).

Table 1. Summary of correlation coefficients and significance of SLE Index with correlates. Statistical significance and strength indicated by *.
S. Sule: None; O. Lamanna: None; K. Jackson: AlgometRx, Inc., 3, 4, 8; S. Ahn: None; T. Ahmed: None; J. Finkel: AlgometRx, 4, 10.



